角质细胞中的螯合素-CMKLR1轴会损害宿主对皮肤金黄色葡萄球菌感染的先天防御能力。
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
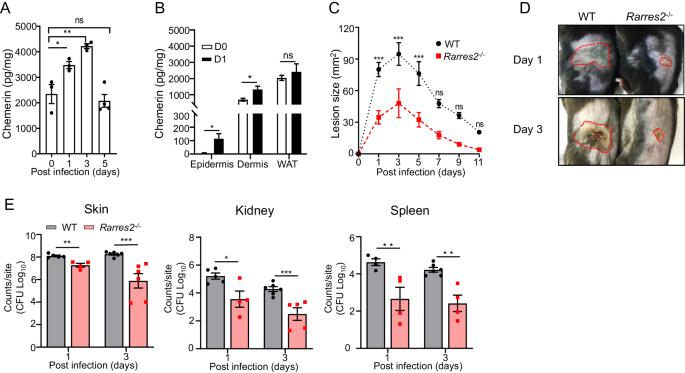
皮肤是金黄色葡萄球菌感染的最常见部位,金黄色葡萄球菌可通过逃避宿主防御而导致各种疾病,包括侵袭性感染和危及生命的感染。然而,人们对促进金黄色葡萄球菌在皮肤中逃避先天性免疫的宿主因素知之甚少。螯合素在皮肤中大量表达,可被来自金黄色葡萄球菌的蛋白酶激活,具有直接杀灭细菌的活性,并通过与其受体 CMKLR1 的相互作用产生免疫调节作用。在这里,我们证明了在小鼠皮肤感染模型中,缺乏螯合素/CMKLR1轴会增加中性粒细胞介导的宿主对金黄色葡萄球菌的防御能力,而螯合素过表达(模拟肥胖个体中高水平的螯合素)则会加剧金黄色葡萄球菌的皮肤感染。从机理上讲,我们发现表达 CMKLR1 的角质形成细胞是螯合素抑制金黄色葡萄球菌诱导的 IL-33 表达的主要靶点,从而导致皮肤中性粒细胞增多和细菌清除能力受损。CMKLR1 信号传导通过抑制小鼠角质形成细胞中 Akt 的活化,特异性地抑制金黄色葡萄球菌细胞壁成分而非分泌蛋白诱导的 IL-33 表达。因此,我们的研究揭示了螯合素/CMKLR1轴的免疫调节作用介导了体内先天性免疫对金葡菌的规避,并可能增加肥胖者对金葡菌感染的易感性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The chemerin-CMKLR1 axis in keratinocytes impairs innate host defense against cutaneous Staphylococcus aureus infection
The skin is the most common site of Staphylococcus aureus infection, which can lead to various diseases, including invasive and life-threatening infections, through evasion of host defense. However, little is known about the host factors that facilitate the innate immune evasion of S. aureus in the skin. Chemerin, which is abundantly expressed in the skin and can be activated by proteases derived from S. aureus, has both direct bacteria-killing activity and immunomodulatory effects via interactions with its receptor CMKLR1. Here, we demonstrate that a lack of the chemerin/CMKLR1 axis increases the neutrophil-mediated host defense against S. aureus in a mouse model of cutaneous infection, whereas chemerin overexpression, which mimics high levels of chemerin in obese individuals, exacerbates S. aureus cutaneous infection. Mechanistically, we identified keratinocytes that express CMKLR1 as the main target of chemerin to suppress S. aureus-induced IL-33 expression, leading to impaired skin neutrophilia and bacterial clearance. CMKLR1 signaling specifically inhibits IL-33 expression induced by cell wall components but not secreted proteins of S. aureus by inhibiting Akt activation in mouse keratinocytes. Thus, our study revealed that the immunomodulatory effect of the chemerin/CMKLR1 axis mediates innate immune evasion of S. aureus in vivo and likely increases susceptibility to S. aureus infection in obese individuals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


