上皮-间质转化特征的增强与不良肿瘤微环境、血管生成和胃癌患者生存率下降有关。
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
上皮-间质转化(EMT)是促进癌细胞转移的重要机制。尽管EMT非常重要,但它在胃癌(GC)患者中的临床意义尚未得到明确证实。为了衡量胃癌中 EMT 的程度,我们采用基因组变异分析对来自两个大型队列的 807 个患者样本进行了评分:TCGA和GSE84437。在这两个队列中,EMT 高的 GC 与较差的总生存率有显著关联(危险比 (HR) = 1.74,p = 0.011 和 HR = 2.01,p = 0.011)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
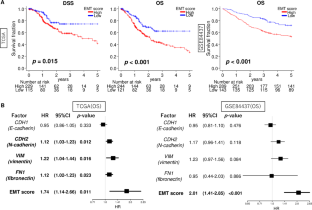
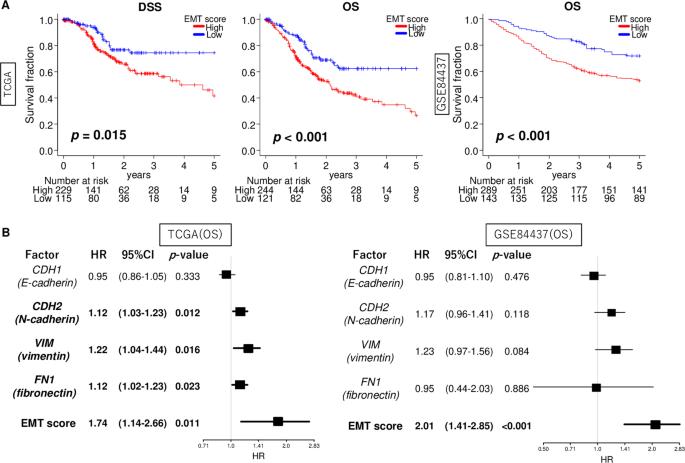
Enhanced epithelial-mesenchymal transition signatures are linked with adverse tumor microenvironment, angiogenesis and worse survival in gastric cancer
Epithelial-mesenchymal transition (EMT) is a crucial mechanism that facilitates cancer cell metastasis. Despite its importance, the clinical significance of EMT in gastric cancer (GC) patients has yet to be clearly demonstrated. For gauging the extent of EMT in GC, we employed gene set variation analysis to score 807 patient samples from two large cohorts: TCGA and GSE84437. In both cohorts, EMT high GC showed a significant association with worse overall survival (hazard ratio (HR) = 1.74, p = 0.011 and HR = 2.01, p < 0.001, respectively). This association was stronger when considering the EMT signature score compared to the individual expressions of EMT-related genes (CDH1, CDH2, VIM, and FN1). While the EMT signature level did not differ among various cancers, high EMT signature specifically correlated with survival in GC alone. Mucinous and diffuse histological types exhibited higher EMT levels compared to others (p < 0.001), and the EMT signature level was correlated with tumor depth and AJCC stage (all p < 0.001). Interestingly, the EMT score was an independent factor for overall and disease-specific survival (multivariate; p = 0.006 and 0.032, respectively). EMT high GC displayed a lower fraction of Th1 cells and a higher fraction of dendritic cells, M1 macrophages and several stromal cells. EMT high GC exhibited an inverse correlation with cell proliferation-related gene sets. While they significantly enriched multiple pro-cancerous gene sets, such as TGF-β signaling, hypoxia, and angiogenesis. The presence of EMT signature in a bulk tumor was linked to TGF-β signaling, hypoxia, and angiogenesis, and was also associated with poorer survival outcomes in GC patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


