铑催化的 2-(1H-吲哚-1-羰基)苯甲酸分子内脱羰基芳基化反应
IF 1.4
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们通过 2-(1H-吲哚-1-羰基)苯甲酸的分子内芳基化,开发了一种氧化还原中性合成异吲哚吲哚酮的方法。该方法有助于生成各种取代的异吲哚吲哚酮,产率从 17% 到 80%不等。我们的机理研究表明了 NaI 的关键作用:碘化阴离子促进了所需异吲哚啉酮的形成,而钠离子则抑制了酰化副产物的形成,从而使异吲哚啉酮能够以可接受的产率选择性地形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
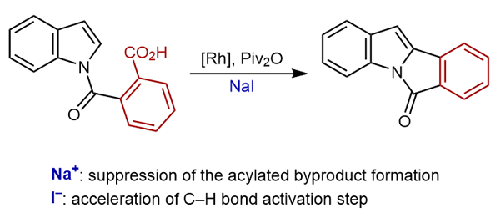
Rhodium-Catalyzed Decarbonylative Intramolecular Arylation of 2-(1H-Indole-1-carbonyl)benzoic Acids
We developed a redox-neutral synthesis of isoindoloindolone via intramolecular arylation of 2-(1H-indole-1-carbonyl)benzoic acids. This protocol facilitates the formation of various substituted isoindoloindolones in yields ranging from 17% to 80%. Our mechanistic investigations indicate the pivotal role of NaI: the iodide anion promotes the formation of the desired isoindoloindolone, and the sodium cation suppresses the formation of acylated byproducts, thereby enabling the selective formation of isoindoloindolones in acceptable yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


