普及艾滋病治疗指南下的长期艾滋病护理结果:25 个国家的回顾性队列研究。
摘要
背景:尽管国家采用通用艾滋病治疗指南提高了抗逆转录病毒疗法(ART)的及时接受率,但对长期护理结果的研究却不足。关于患者自然减员、病毒载量(VL)监测以及开始接受艾滋病治疗后 24 个月和 36 个月的病毒抑制(VS),来自真实世界服务提供环境的数据很少:在这项回顾性队列分析中,我们使用了国际艾滋病流行病学评估数据库(IeDEA)联盟亚太、中非、东非、中美/南美和北美地区 25 个国家的观察数据,这些数据是 2012 年至 2018 年间,在国家采用普遍治疗指南之前 24 个月至之后 12 个月期间,抗逆转录病毒疗法(ART)未接受治疗且入院时年龄≥15 岁的患者的数据。我们使用竞争风险回归法估算了在采用指南前后加入护理服务的患者在入院后 12、24 和 36 个月失去诊所(CI-LTC)的粗累计发生率。通过特定病因的 Cox 比例危险回归模型估算了入院后每个时间点与指南变更相关的 LTC 危险比。改良泊松回归用于估算抗逆转录病毒疗法开始后 12、24 和 36 个月的保留率、VL 监测和 VS 的相对风险。共有 66,963 名患者在 109 家诊所接受了 HIV 治疗,入院后随访时间≥12 个月(46,484 人 [69.4%] 在指南采用前入院,20,479 人 [30.6%] 在指南采用后入院)。一半以上(54.9%)为女性,年龄中位数为 34 岁(四分位数间距 [IQR]:27 岁至 43 岁)。平均随访时间为 51 个月(标准差:17 个月;范围:12 至 110 个月)。在指南采用前登记的患者中,粗 CI-LTC 在登记后 12 个月为 23.8%(95% 置信区间 [95% CI] 23.4,24.2),24 个月为 31.0%(95% CI [30.6,31.5]),36 个月为 37.2%(95% [CI 36.8,37.7])。在对性别、年龄组、入院 CD4、诊所地点和类型以及国家收入水平进行调整后,在指南采用后入院并开始抗逆转录病毒疗法与 12 个月时 LTC 风险增加有关(调整后风险比 [aHR] 1.25 [95% CI 1.08, 1.44];p = 0.003)、24 个月(aHR 1.38 [95% CI 1.19, 1.59];p < .001)和 36 个月(aHR 1.34 [95% CI 1.18, 1.53],p < .001)与采用指南前的入组相比,在随访结束时无开始抗逆转录病毒疗法记录的患者中,前后无差异。在开始接受抗逆转录病毒疗法后保留下来的患者中,VL 监测率较低,只有在开始接受抗逆转录病毒疗法 12 个月后,采用指南才会带来边际改善。在接受 VL 监测的患者中,指南采用前(86.0% 至 88.8%)和指南采用后(86.2% 至 90.3%)每个时间点的 VS 均较高,与指南采用没有实质性差异。研究的局限性包括现实世界服务提供数据的滞后性和护理结果的潜在不确定性,以及可能缺乏对本分析所包括的IeDEA地点和地区之外的普遍性:在这项研究中,采用普及艾滋病治疗指南与抗逆转录病毒疗法启动后随访 36 个月的留存率较低有关,留存患者的 VL 监测或 VS 变化不大。监测长期的 HIV 护理结果对于识别和解决流失原因及 HIV 护理质量差距仍然至关重要。
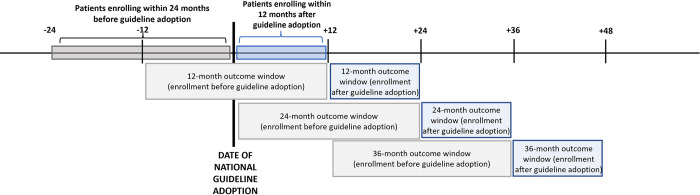


Background: While national adoption of universal HIV treatment guidelines has led to improved, timely uptake of antiretroviral therapy (ART), longer-term care outcomes are understudied. There is little data from real-world service delivery settings on patient attrition, viral load (VL) monitoring, and viral suppression (VS) at 24 and 36 months after HIV treatment initiation.
Methods and findings: For this retrospective cohort analysis, we used observational data from 25 countries in the International epidemiology Databases to Evaluate AIDS (IeDEA) consortium's Asia-Pacific, Central Africa, East Africa, Central/South America, and North America regions for patients who were ART naïve and aged ≥15 years at care enrollment between 24 months before and 12 months after national adoption of universal treatment guidelines, occurring 2012 to 2018. We estimated crude cumulative incidence of loss-to-clinic (CI-LTC) at 12, 24, and 36 months after enrollment among patients enrolling in care before and after guideline adoption using competing risks regression. Guideline change-associated hazard ratios of LTC at each time point after enrollment were estimated via cause-specific Cox proportional hazards regression models. Modified Poisson regression was used to estimate relative risks of retention, VL monitoring, and VS at 12, 24, and 36 months after ART initiation. There were 66,963 patients enrolling in HIV care at 109 clinics with ≥12 months of follow-up time after enrollment (46,484 [69.4%] enrolling before guideline adoption and 20,479 [30.6%] enrolling afterwards). More than half (54.9%) were females, and median age was 34 years (interquartile range [IQR]: 27 to 43). Mean follow-up time was 51 months (standard deviation: 17 months; range: 12, 110 months). Among patients enrolling before guideline adoption, crude CI-LTC was 23.8% (95% confidence interval [95% CI] 23.4, 24.2) at 12 months, 31.0% (95% CI [30.6, 31.5]) at 24 months, and 37.2% (95% [CI 36.8, 37.7]) at 36 months after enrollment. Adjusting for sex, age group, enrollment CD4, clinic location and type, and country income level, enrolling in care and initiating ART after guideline adoption was associated with increased hazard of LTC at 12 months (adjusted hazard ratio [aHR] 1.25 [95% CI 1.08, 1.44]; p = 0.003); 24 months (aHR 1.38 [95% CI 1.19, 1.59]; p < .001); and 36 months (aHR 1.34 [95% CI 1.18, 1.53], p < .001) compared with enrollment before guideline adoption, with no before-after differences among patients with no record of ART initiation by end of follow-up. Among patients retained after ART initiation, VL monitoring was low, with marginal improvements associated with guideline adoption only at 12 months after ART initiation. Among those with VL monitoring, VS was high at each time point among patients enrolling before guideline adoption (86.0% to 88.8%) and afterwards (86.2% to 90.3%), with no substantive difference associated with guideline adoption. Study limitations include lags in and potential underascertainment of care outcomes in real-world service delivery data and potential lack of generalizability beyond IeDEA sites and regions included in this analysis.
Conclusions: In this study, adoption of universal HIV treatment guidelines was associated with lower retention after ART initiation out to 36 months of follow-up, with little change in VL monitoring or VS among retained patients. Monitoring long-term HIV care outcomes remains critical to identify and address causes of attrition and gaps in HIV care quality.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


