具有三聚呋喃骨架的高能材料的热化学和分解行为
IF 2
4区 工程技术
Q3 CHEMISTRY, APPLIED
引用次数: 0
摘要
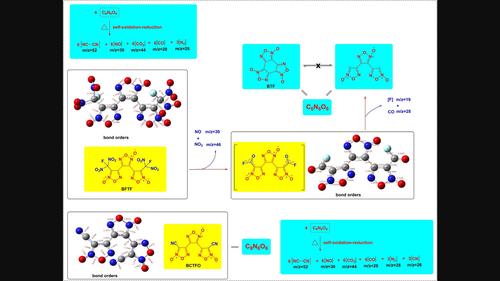
三聚呋喃具有结构紧凑、形成焓高的特点,是构建高能物质的理想分子骨架。为了探索和比较具有串联三聚呋喃分子骨架的高能材料的热行为,我们首次系统研究了3,4-双(3-氟二硝基甲基呋喃-4-基)呋喃(BFTF)、3,4-双(3-氰基呋喃)氧化呋喃(BCTFO)和苯并三呋喃(BTF)的热化学行为和分解机理。根据 DSC-TG 实验的研究结果,两种取代的呋喃类高能化合物(BCTFO 和 BFTF)在 240 ℃ 左右都表现出较低的熔点和复杂的热分解行为,而未取代的呋喃类化合物(BTF)的熔点要高得多。根据原位傅立叶变换红外光谱法和 DSC-TG-FTIR-MS 四重技术等串联技术的实验结果,提出了它们的详细分解机理,表明取代基的裂解会引发 BFTF 的分解,而三聚呋喃骨架的分解几乎与 BCTFO 中取代基的裂解同步发生。线性和环状三聚呋喃的自氧化还原反应会产生类似的分解碎片小分子产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thermal chemistry and decomposition behaviors of energetic materials with trimerizing furoxan skeleton
Trimerizing furoxans are ideal molecular skeletons for the construction of high energetic substances due to their compact structures and high enthalpy of formations. To explore and compare the thermal behaviors of energetic materials with tandem trimerizing furoxan molecular skeleton, we reported the first systematic research on the thermochemical behaviors and decomposition mechanism of 3,4‐bis(3‐fluorodinitromethylfuroxan‐4‐yl)furoxan (BFTF), 3,4‐bis(3‐cyanofurazan)furazan oxide (BCTFO) and benzotrifuroxan (BTF). According to the research results of the DSC‐TG experiments, both the substituted furoxan based energetic compounds (BCTFO and BFTF) exhibited low melting points and complicated thermal decomposition behaviors around 240 °C, while the melting point of unsubstituted furoxan (BTF) was much higher. Their detailed decomposition mechanisms were proposed based on the experimental results through tandem techniques including in‐situ FTIR spectroscopy method and DSC‐TG‐FTIR‐MS quadruple technology, which indicated that the cleavage of substituent would trigger the decompositions of BFTF and the decomposition of trimerizing furoxan skeletons almost synchronous occurrence with substituents in BCTFO. The self‐oxidation‐reduction of the linear and annular trimerizing furoxans lead to similar decomposition fragmented small molecule products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Propellants, Explosives, Pyrotechnics
工程技术-工程:化工
CiteScore
4.20
自引率
16.70%
发文量
235
审稿时长
2.7 months
期刊介绍:
Propellants, Explosives, Pyrotechnics (PEP) is an international, peer-reviewed journal containing Full Papers, Short Communications, critical Reviews, as well as details of forthcoming meetings and book reviews concerned with the research, development and production in relation to propellants, explosives, and pyrotechnics for all applications. Being the official journal of the International Pyrotechnics Society, PEP is a vital medium and the state-of-the-art forum for the exchange of science and technology in energetic materials. PEP is published 12 times a year.
PEP is devoted to advancing the science, technology and engineering elements in the storage and manipulation of chemical energy, specifically in propellants, explosives and pyrotechnics. Articles should provide scientific context, articulate impact, and be generally applicable to the energetic materials and wider scientific community. PEP is not a defense journal and does not feature the weaponization of materials and related systems or include information that would aid in the development or utilization of improvised explosive systems, e.g., synthesis routes to terrorist explosives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


