参与 U-PGx PREPARE 研究的奥地利肾移植受者在药物基因组学指导下接受他克莫司治疗的成本效益分析
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
摘要
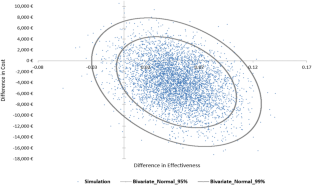
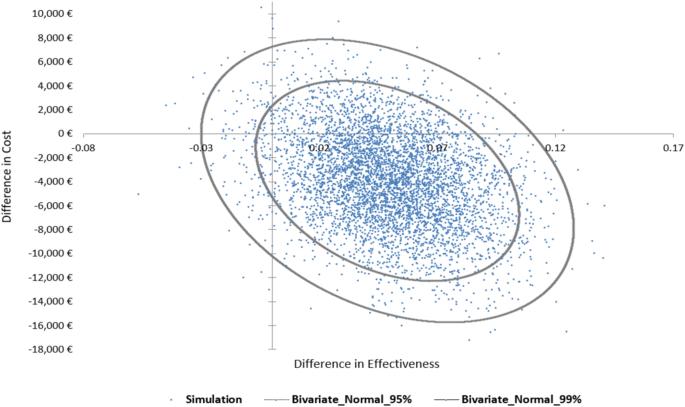
慢性肾脏病(CKD)是一个全球性的健康问题。肾衰竭患者可能会接受肾移植手术(KTX),并服用免疫抑制剂,即他克莫司。他克莫司的疗效和毒性因人而异。本研究调查了参与 PREPARE UPGx 研究的奥地利 KTX 患者在药物基因组学(PGx)指导下使用他克莫司治疗与传统方法相比的成本效益。治疗效果根据平均存活率确定,效用值根据视觉模拟量表评分确定。同时还计算了增量成本效益比。结果发现,与对照组相比,PGx 指导治疗组具有成本效益,降低了成本(减少了 3902 欧元),减少了 6% 的住院天数,降低了药物不良事件的风险。PGx 指导治疗组的平均 QALYs 为 0.900(95% CI:0.862-0.936),而其他治疗组的平均 QALYs 为 0.851(95% CI:0.814-0.885)。总之,在奥地利的医疗环境中,PGx 指导下的他克莫司治疗是一种节约成本的选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cost-utility analysis of pharmacogenomics-guided tacrolimus treatment in Austrian kidney transplant recipients participating in the U-PGx PREPARE study
Chronic kidney disease (CKD) is a global health issue. Kidney failure patients may undergo a kidney transplantation (KTX) and prescribed an immunosuppressant medication i.e., tacrolimus. Tacrolimus’ efficacy and toxicity varies among patients. This study investigates the cost-utility of pharmacogenomics (PGx) guided tacrolimus treatment compared to the conventional approach in Austrian patients undergone KTX, participating in the PREPARE UPGx study. Treatment’s effectiveness was determined by mean survival, and utility values were based on a Visual Analog Scale score. Incremental Cost-Effectiveness Ratio was also calculated. PGx-guided treatment arm was found to be cost-effective, resulting in reduced cost (3902 euros less), 6% less hospitalization days and lower risk of adverse drug events compared to the control arm. The PGx-guided arm showed a mean 0.900 QALYs (95% CI: 0.862–0.936) versus 0.851 QALYs (95% CI: 0.814–0.885) in the other arm. In conclusion, PGx-guided tacrolimus treatment represents a cost-saving option in the Austrian healthcare setting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


