腺相关病毒介导的曲妥珠单抗向中枢神经系统输送治疗人类表皮生长因子受体 2+ 脑转移瘤的药物
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
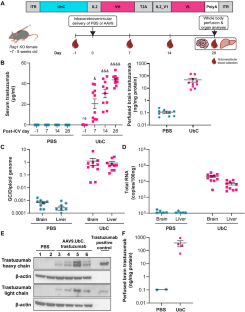
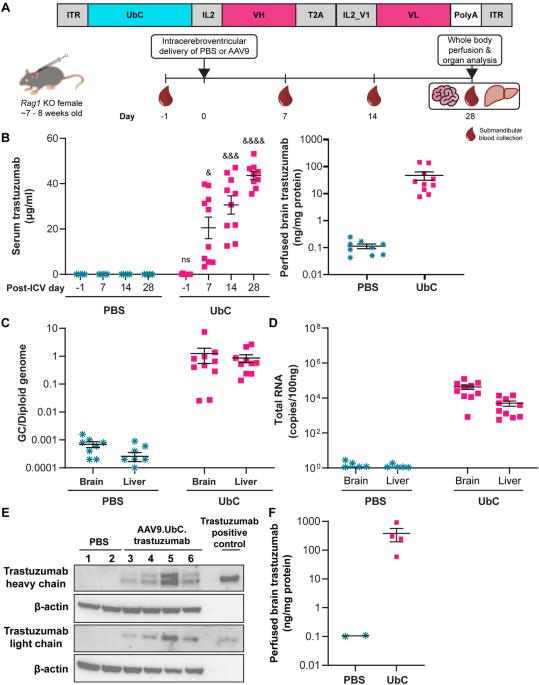
曲妥珠单抗可提高 HER2+ 乳腺癌患者的总生存率,但其在脑脊液中的半衰期较短(约 2-4 天),而且给药限制了针对 HER2+ 中枢神经系统(CNS)疾病的能力。我们开发了一种表达经过密码子优化的泛素 C (UbC) 启动子驱动的曲妥珠单抗序列(AAV9.UbC.trastuzumab)的腺相关病毒(AAV)载体,用于鞘内给药。在成年 Rag1 基因敲除小鼠和恒河猴等非人灵长类动物(NHPs)脑室内(ICV)或椎管内(ICM)注射 AAV9.UbC.trastuzumab 后,对转基因表达进行了评估。在使用 HER2+ 乳腺癌细胞系(BT-474、MDA-MB-453)的脑异种移植中评估了抗肿瘤效果。单次 ICV 注射 AAV9.UbC.曲妥珠单抗(1 × 1011 载体基因组拷贝 [GC]/小鼠)后,在 Rag1 基因敲除小鼠的脑匀浆中检测到转基因表达,在乳腺癌脑转移异种移植模型中抑制了肿瘤进展。在 NHPs 中,AAV9.UbC.曲妥珠单抗(3 × 1013 GC/只)的 ICM 输送耐受性良好(36-37 天存活期),在中枢神经系统组织和脑脊液中的转基因表达水平足以诱导 MDA-MB-453 脑异种移植的肿瘤完全缓解。AAV9 的临床安全性已得到证实,因此这种基因疗法可能是针对 HER2 + 中枢神经系统恶性肿瘤的一种可行方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Adeno-associated virus-mediated trastuzumab delivery to the central nervous system for human epidermal growth factor receptor 2+ brain metastasis
Trastuzumab improves overall survival for HER2+ breast cancer, but its short half-life in the cerebrospinal fluid (~2–4 days) and delivery limitations restrict the ability to target HER2+ central nervous system (CNS) disease. We developed an adeno-associated virus (AAV) vector expressing a codon-optimized, ubiquitin C (UbC)-promoter-driven trastuzumab sequence (AAV9.UbC.trastuzumab) for intrathecal administration. Transgene expression was evaluated in adult Rag1 knockout mice and rhesus nonhuman primates (NHPs) after a single intracerebroventricular (ICV) or intra-cisterna magna (ICM) AAV9.UbC.trastuzumab injection, respectively, using real-time PCR, ELISA, Western blot, in situ hybridization, single-nucleus RNA sequencing, and liquid chromatography-mass spectrometry; antitumor efficacy was evaluated in brain xenografts using HER2+ breast cancer cell lines (BT-474, MDA-MB-453). Transgene expression was detected in brain homogenates of Rag1 knockout mice following a single ICV injection of AAV9.UbC.trastuzumab (1 × 1011 vector genome copies [GC]/mouse) and tumor progression was inhibited in xenograft models of breast-to-brain metastasis. In NHPs, ICM delivery of AAV9.UbC.trastuzumab (3 × 1013 GC/animal) was well tolerated (36–37 days in-life) and resulted in transgene expression in CNS tissues and cerebrospinal fluid at levels sufficient to induce complete tumor remission in MDA-MB-453 brain xenografts. With AAV9’s proven clinical safety record, this gene therapy may represent a viable approach for targeting HER2 + CNS malignancies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


