AAV8 中枢神经系统注射后的脱落分析显示,小鼠体内的病毒 DNA 已破碎,但没有功能性 AAV 粒子的迹象。
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
腺相关病毒(AAV)因其应用范围广泛而常用于科学领域。然而,AAV脱落,即病毒从宿主生物体中释放出来,会影响基于AAV方法的安全性。越来越多的权威机构要求在临床试验中对载体脱落进行鉴定。最近,转导实验动物的脱落也引起了人们对其废品必要处置措施的关注。然而,国际上还没有针对 AAV 脱落废物的明确规定。深入了解脱落动态对帮助有关当局制定适当的法规越来越重要。迄今为止,人们对小鼠体内 AAV 载体脱落的了解非常有限。此外,还缺少对小鼠体内功能性脱落 AAV 粒子的确认。因此,我们检测了中枢神经系统注射 AAV2/8 后小鼠的粪便、尿液和唾液。通过 qPCR,我们发现在注射后的 4 天内都存在病毒 DNA 片段。为了检测 AAV 的功能性,我们进行了巢式 PCR 检测,结果发现除了两个粪便样本外,其他样本都检测不到全长病毒基因组。此外,功能性感染检测也没有发现完整的 AAV 颗粒。我们的研究结果将为小鼠的脱落数据提供依据,以帮助制定有关 AAV 脱落的生物安全法规。本文章由计算机程序翻译,如有差异,请以英文原文为准。
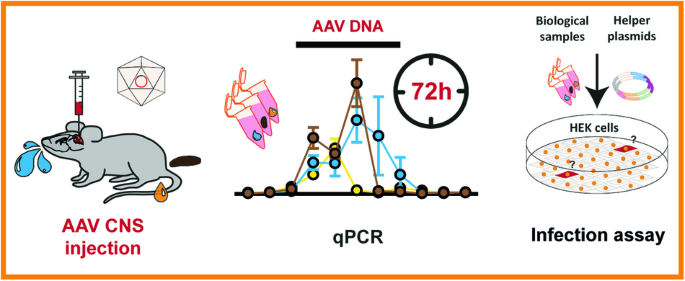
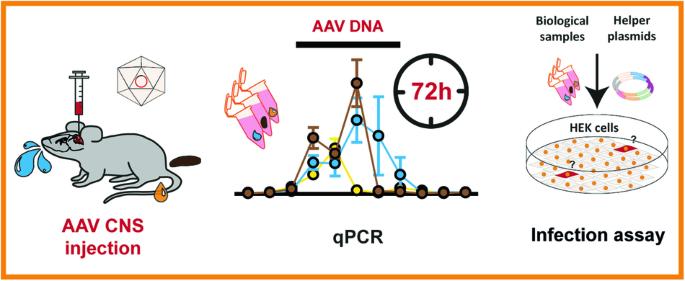
A shedding analysis after AAV8 CNS injection revealed fragmented viral DNA without evidence of functional AAV particles in mice
Adeno-associated viruses (AAV) are commonly used in the scientific field due to their diverse application range. However, AAV shedding, the release of virions from the host organism, can impact the safety of AAV-based approaches. An increasing number of authorities require the characterization of vector shedding in clinical trials. Recently, shedding of transduced laboratory animals has also gained attention regarding the necessary disposal measures of their waste products. However, no explicit international regulations for AAV-shedding waste exist. Generating insights into shedding dynamics becomes increasingly relevant to help authorities develop adequate regulations. To date, knowledge of AAV vector shedding in mice is very limited. Moreover, confirmation of functional shed AAV particles in mice is missing. Therefore, we examined feces, urine, and saliva of mice after CNS injection with AAV2/8. It revealed the presence of viral DNA fragments via qPCR for up to 4 days after injection. To examine AAV functionality we performed nested PCR and could not detect full-length viral genomes in any but two collected feces samples. Furthermore, a functional infection assay did not reveal evidence of intact AAV particles. Our findings are supposed to contribute murine shedding data as a foundation to help establish still lacking adequate biosafety regulations in the context of AAV shedding.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


