通过光化学环化法合成消旋肌苷 B 及其类似物
IF 1.4
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过光化学环化反应合成生物碱消旋莫辛 B 的简单方法已经开发出来,无需任何试剂,总产率为 33%。这种方法无需任何试剂即可实现转化,因此具有可持续性和环保性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
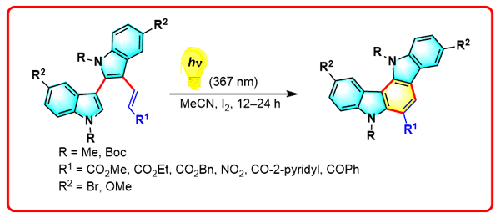
Synthesis of Racemosin B and Its Analogues by a Photochemical Cyclization
A simple approach for the synthesis of the alkaloid racemosin B has been developed through a photochemical cyclization reaction with a 33% overall yield without any reagents. This method is sustainable and environmentally friendly because no reagents are required to effect the transformation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


