在温和条件下有机光氧化羰基化阿伦迭嗪
IF 1.7
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
多种羰基化合物的模块化合成是有机合成的核心。我们开发了一种光氧化羰基化的优化方案,该方案以间苯二甲酸吖啶鎓为光催化剂,可在较温和的条件下操作。在 20 巴 CO 和 20 °C 的条件下,阿伦迭氮盐被转化为苯甲酸盐(与醇)、苯甲酸(与水)、苯甲酰胺(与胺)和氯化物(与 1-丁基-3-甲基咪唑氯化物)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
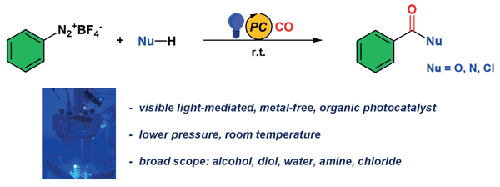
Organic Photoredox Carbonylation of Arenediazonium under Mild Conditions
The modular synthesis of diverse carbonyl compounds is at the heart of organic synthesis. An optimized protocol for photoredox carbonylation was developed that operates under milder conditions with mesitylacridinium as a photocatalyst. Arenediazonium salts were converted into benzoates (with alcohols), benzoic acids (with water), benzamides (with amines), and chlorides (with 1-butyl-3-methylimidazolium chloride) at 20 bar CO and 20 °C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synlett
化学-有机化学
CiteScore
3.40
自引率
5.00%
发文量
369
审稿时长
1 months
期刊介绍:
SYNLETT is an international journal reporting research results and current trends in chemical synthesis in short personalized reviews and preliminary communications. It covers all fields of scientific endeavor that involve organic synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines and offers the possibility to publish scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


