探索异雌甾醇的吡啶酰-腙衍生物的两种不同结晶途径:真正的和假对称的同手性图案
IF 2.1
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
异雌甾二醇的吡啶酮-腙衍生物分子中同时存在两个片段,即一个手性异雌甾二醇分子和一个非手性吡啶酮-腙分子,这使得它们的晶体中出现了两种不同的超分子结构,即真正的同手性结构和假对称同手性结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
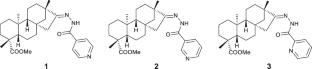
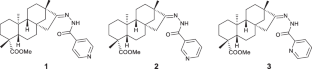
Exploring two distinct crystallization pathways of pyridinoyl-hydrazone derivatives of isosteviol: true and pseudosymmetric homochiral motifs
The coexistence of two fragments, a chiral isosteviol moiety and an achiral pyridinoyl-hydrazone moiety, in the molecules of pyridinoyl-hydrazone derivatives of isosteviol results in the realization of two distinct supramolecular motifs in their crystals, namely, a true homochiral motif and a pseudosymmetric homochiral motif.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structural Chemistry
化学-化学综合
CiteScore
3.80
自引率
11.80%
发文量
227
审稿时长
3.7 months
期刊介绍:
Structural Chemistry is an international forum for the publication of peer-reviewed original research papers that cover the condensed and gaseous states of matter and involve numerous techniques for the determination of structure and energetics, their results, and the conclusions derived from these studies. The journal overcomes the unnatural separation in the current literature among the areas of structure determination, energetics, and applications, as well as builds a bridge to other chemical disciplines. Ist comprehensive coverage encompasses broad discussion of results, observation of relationships among various properties, and the description and application of structure and energy information in all domains of chemistry.
We welcome the broadest range of accounts of research in structural chemistry involving the discussion of methodologies and structures,experimental, theoretical, and computational, and their combinations. We encourage discussions of structural information collected for their chemicaland biological significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


