转基因原生动物是否有资格获得 ATMP 地位?关于溶瘤原生动物候选药物的法律分类
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
犬新孢子虫(Neospora caninum)是一种细胞内原生动物,会影响多种动物物种。它对人类不具致病性,能感染和溶解多种细胞并刺激免疫系统,因此是肿瘤学领域一种有趣的候选药物。N. caninum 的内在溶瘤特性已在多个临床前模型中得到证实。此外,还可以对其进行改造,以提高其安全性和/或对癌细胞的疗效。在本研究中,我们提出了这种新型生物候选药物的法律分类以及改造的影响,特别是在其基因组中整合自杀基因、删除允许其在健康细胞中繁殖的基因和/或插入编码治疗蛋白的基因。未经改造的金线虫可归类为生物药品,而旨在提高其安全性的改造则将其归类为体细胞疗法药品,而旨在提高其疗效或兼具安全性和疗效的改造则将其归类为基因疗法药品。这种分类非常重要,因为它决定了适用于临床前开发的指导方针。这些准则繁多而复杂,我们将重点放在开发未来医药产品所需的关键要求上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
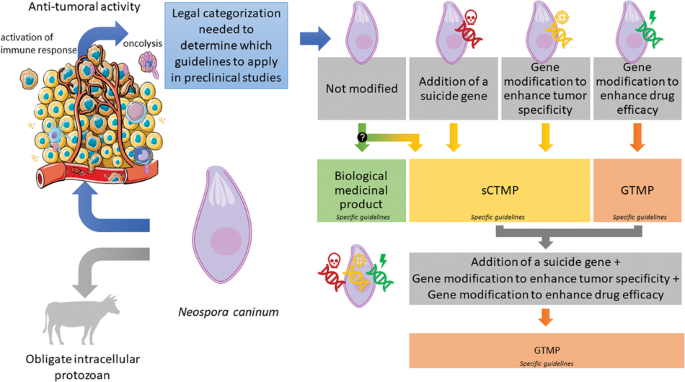
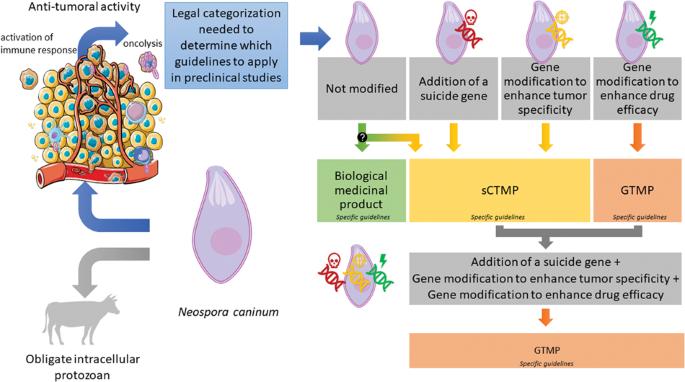
Are genetically modified protozoa eligible for ATMP status? Concerning the legal categorization of an oncolytic protozoan drug candidate
Neospora caninum is an obligate intracellular protozoan that affects several animal species. It is not pathogenic for humans, and its ability to infect and lyse a variety of cells and stimulate the immune system makes it an interesting drug candidate in oncology. The intrinsic oncolytic properties of N. caninum have been confirmed in several preclinical models. Moreover, it can be modified to improve its safety and/or efficacy against cancer cells. In this study, we propose the legal categorization of this new biological drug candidate and the impact of modifications, notably the integration of a suicide gene, the deletion of a gene allowing its multiplication in healthy cells, and/or the insertion of a gene coding for a therapeutic protein into its genome. When unmodified, N. caninum can be categorized as a biological medicinal product, whereas modifications aimed at increasing its safety classify it as a Somatic Cell Therapy Medicinal Product, and modifications aiming to increase its efficacy or both safety and efficacy make it as a Gene Therapy Medicinal Product. This categorization is fundamental because it determines the guidelines applicable for preclinical development. These guidelines being numerous and complex, we have focused on the key requirements necessary for the development of the future medicinal product.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


