缺氧激活的XBP1s招募HDAC2-EZH2参与ΔNp63α表达的表观遗传抑制并促进乳腺癌转移,而与HIF1α无关。
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
缺氧是癌症发展的一个标志。然而,低氧促进肿瘤转移的分子机制尚未完全清楚。在这项研究中,我们证明缺氧通过抑制ΔNp63α以一种与HIF1α无关的方式促进乳腺癌转移。我们发现,缺氧激活的XBP1s与HDAC2和EZH2形成稳定的抑制蛋白复合物,抑制ΔNp63α的转录。值得注意的是,在常氧条件下,H3K27ac主要占据ΔNp63启动子,而在缺氧条件下,H3K27me3则占据启动子。我们的研究表明,XBP1s与ΔNp63启动子结合,招募HDAC2和EZH2,促进H3K27ac向H3K27me3的转换。药物抑制或敲除 HDAC2 或 EZH2 会导致 H3K27ac 增加,同时 H3K27me3 减少,并恢复被缺氧抑制的 ΔNp63α 表达,从而抑制细胞迁移。此外,药物抑制 IRE1α(而非 HIF1α)可上调体外的 ΔNp63α 表达,并抑制体内的肿瘤转移。临床分析表明,p63 表达的降低与 XBP1、HDAC2 或 EZH2 表达的升高相关,并与人类乳腺癌患者的总生存率低有关。这些结果表明,缺氧激活的 XBP1s 可调节抑制 ΔNp63α 的表观遗传学程序,从而促进乳腺癌转移,而不依赖于 HIF1α,并为靶向 XBP1s/HDAC2/EZH2-ΔNp63α 轴作为治疗乳腺癌转移的一种可能策略提供了分子基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
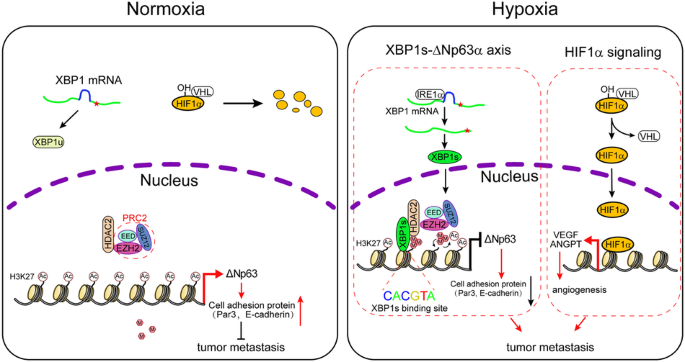
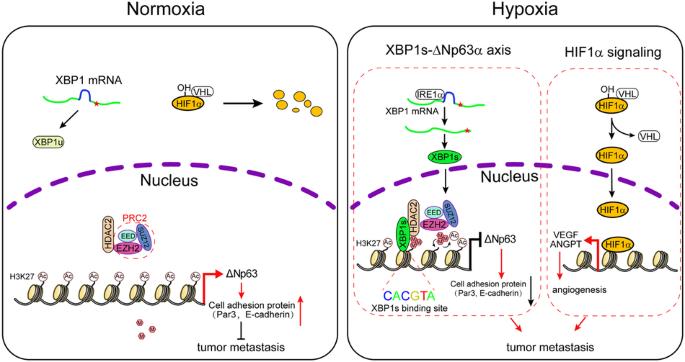
Hypoxia-activated XBP1s recruits HDAC2-EZH2 to engage epigenetic suppression of ΔNp63α expression and promote breast cancer metastasis independent of HIF1α
Hypoxia is a hallmark of cancer development. However, the molecular mechanisms by which hypoxia promotes tumor metastasis are not fully understood. In this study, we demonstrate that hypoxia promotes breast cancer metastasis through suppression of ΔNp63α in a HIF1α-independent manner. We show that hypoxia-activated XBP1s forms a stable repressor protein complex with HDAC2 and EZH2 to suppress ΔNp63α transcription. Notably, H3K27ac is predominantly occupied on the ΔNp63 promoter under normoxia, while H3K27me3 on the promoter under hypoxia. We show that XBP1s binds to the ΔNp63 promoter to recruit HDAC2 and EZH2 in facilitating the switch of H3K27ac to H3K27me3. Pharmacological inhibition or the knockdown of either HDAC2 or EZH2 leads to increased H3K27ac, accompanied by the reduced H3K27me3 and restoration of ΔNp63α expression suppressed by hypoxia, resulting in inhibition of cell migration. Furthermore, the pharmacological inhibition of IRE1α, but not HIF1α, upregulates ΔNp63α expression in vitro and inhibits tumor metastasis in vivo. Clinical analyses reveal that reduced p63 expression is correlated with the elevated expression of XBP1, HDAC2, or EZH2, and is associated with poor overall survival in human breast cancer patients. Together, these results indicate that hypoxia-activated XBP1s modulates the epigenetic program in suppression of ΔNp63α to promote breast cancer metastasis independent of HIF1α and provides a molecular basis for targeting the XBP1s/HDAC2/EZH2-ΔNp63α axis as a putative strategy in the treatment of breast cancer metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


