通过α-酮戊二酸和mTORC1信号转导,靶向PHGDH可逆转肿瘤相关巨噬细胞的免疫抑制表型。
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
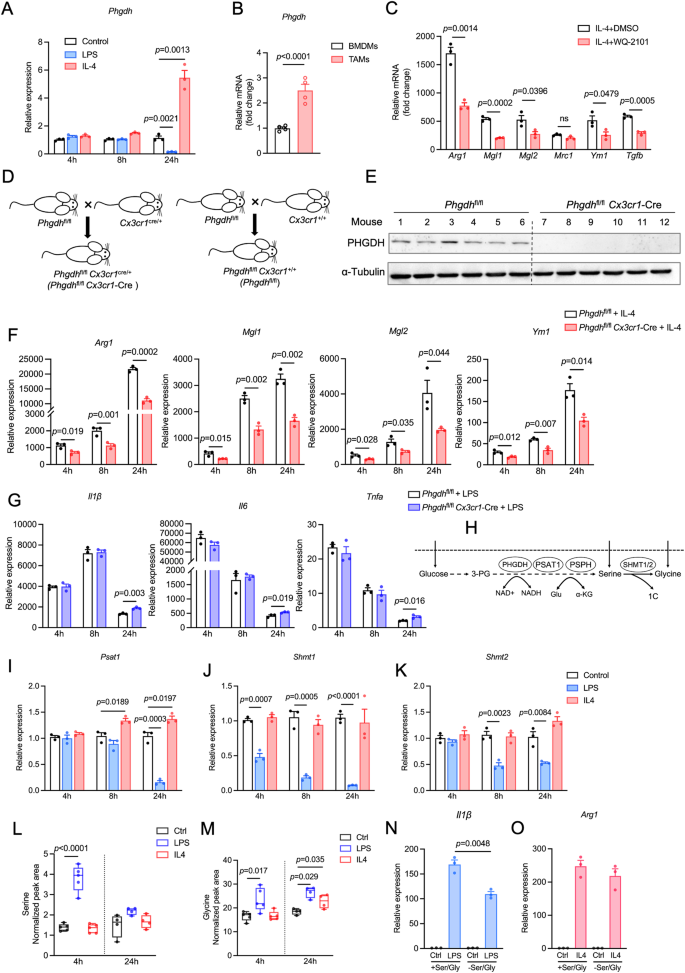
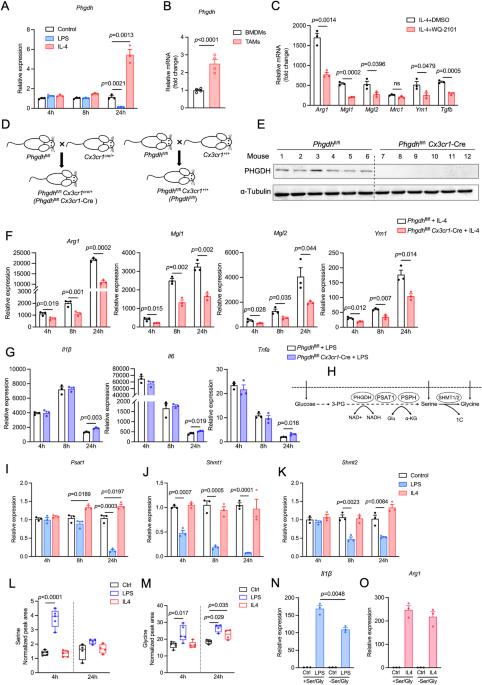
磷酸甘油脱氢酶(PHGDH)已成为癌细胞、淋巴细胞和内皮细胞合成大分子、中和氧化应激以及调节甲基化反应的关键因素。然而,人们对 PHGDH 在肿瘤相关巨噬细胞(TAMs)中的作用知之甚少。在这里,我们发现T辅助2(Th2)细胞因子白细胞介素-4和肿瘤调节介质会上调巨噬细胞中PHGDH的表达,并促进免疫抑制性M2巨噬细胞的活化和增殖。PHGDH 的缺失会破坏细胞代谢和线粒体呼吸,而这对于免疫抑制巨噬细胞来说是必不可少的。从机理上讲,PHGDH 介导的丝氨酸生物合成促进了 α-酮戊二酸的产生,从而激活了 mTORC1 信号传导,有助于在肿瘤微环境中维持 M2 样巨噬细胞表型。遗传性消减肿瘤小鼠巨噬细胞中的 PHGDH 会导致肿瘤生长减慢、TAM 浸润减少、M2 样 TAM 表型向 M1 样表型转变、PD-L1 表达下调以及抗肿瘤 T 细胞免疫增强。我们的研究为进一步探索 PHGDH 作为潜在靶点以对抗 TAM 介导的免疫抑制和阻碍肿瘤进展提供了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting PHGDH reverses the immunosuppressive phenotype of tumor-associated macrophages through α-ketoglutarate and mTORC1 signaling
Phosphoglycerate dehydrogenase (PHGDH) has emerged as a crucial factor in macromolecule synthesis, neutralizing oxidative stress, and regulating methylation reactions in cancer cells, lymphocytes, and endothelial cells. However, the role of PHGDH in tumor-associated macrophages (TAMs) is poorly understood. Here, we found that the T helper 2 (Th2) cytokine interleukin-4 and tumor-conditioned media upregulate the expression of PHGDH in macrophages and promote immunosuppressive M2 macrophage activation and proliferation. Loss of PHGDH disrupts cellular metabolism and mitochondrial respiration, which are essential for immunosuppressive macrophages. Mechanistically, PHGDH-mediated serine biosynthesis promotes α-ketoglutarate production, which activates mTORC1 signaling and contributes to the maintenance of an M2-like macrophage phenotype in the tumor microenvironment. Genetic ablation of PHGDH in macrophages from tumor-bearing mice results in attenuated tumor growth, reduced TAM infiltration, a phenotypic shift of M2-like TAMs toward an M1-like phenotype, downregulated PD-L1 expression and enhanced antitumor T-cell immunity. Our study provides a strong basis for further exploration of PHGDH as a potential target to counteract TAM-mediated immunosuppression and hinder tumor progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


