NLRP3 炎症小体激活与 NETosis 相互正向调节并加剧促炎反应:抑制 NETosis 对痤疮皮肤炎症治疗的意义
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
炎症体是一种多蛋白复合物,参与宿主对病原体感染的免疫反应。因此,炎性体参与了许多疾病的治疗,如痤疮。最近的研究表明,中性粒细胞死亡的一种类型--NETosis 是由细菌感染诱发的,并与糖尿病患者伤口延迟愈合等炎症性疾病有关。然而,炎性体和 NETosis 在炎症性疾病发病机制中的关系还没有得到很好的研究。在这项研究中,我们确定了痤疮丙酸杆菌诱导的皮肤炎症是否会诱导NETosis,以及在痤疮皮肤炎症小鼠模型中,核苷酸结合域、富亮氨酸家族和含吡啶域-3(NLRP3)炎性体的激活是否是诱导NETosis的关键因素之一。我们发现,在痤疮丙酸杆菌诱导的小鼠皮肤炎症中,NETosis 被诱导,而抑制 NETosis 可改善痤疮丙酸杆菌诱导的皮肤炎症。此外,我们的研究结果表明,抑制炎性体的活化可抑制小鼠皮肤中的NETosis诱导。这些结果表明,炎性体和NETosis可以相互作用,诱导痤疮诱导的皮肤炎症,并表明靶向NETosis可能是治疗炎性体介导的疾病以及NETosis相关疾病的一种潜在方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
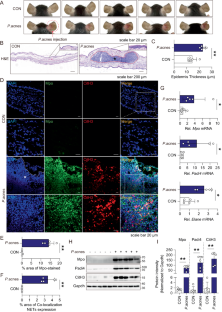
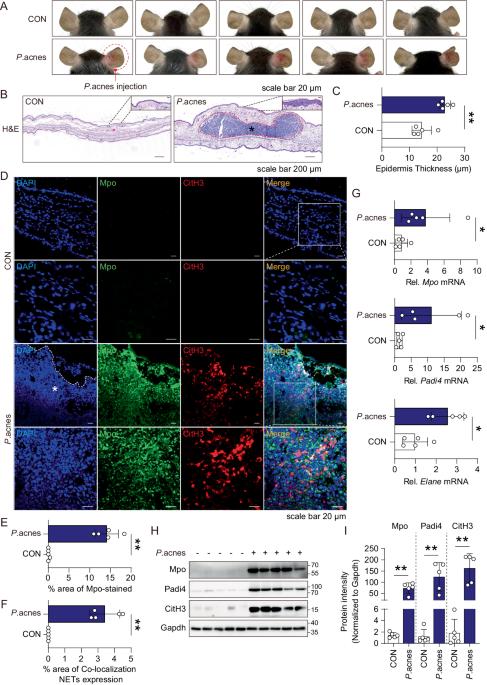
NLRP3 inflammasome activation and NETosis positively regulate each other and exacerbate proinflammatory responses: implications of NETosis inhibition for acne skin inflammation treatment
Inflammasomes are multiprotein complexes involved in the host immune response to pathogen infections. Thus, inflammasomes participate in many conditions, such as acne. Recently, it was shown that NETosis, a type of neutrophil cell death, is induced by bacterial infection and is involved in inflammatory diseases such as delayed wound healing in patients with diabetes. However, the relationship between inflammasomes and NETosis in the pathogenesis of inflammatory diseases has not been well studied. In this study, we determined whether NETosis is induced in P. acnes-induced skin inflammation and whether activation of the nucleotide-binding domain, leucine-rich family, and pyrin domain-containing-3 (NLRP3) inflammasome is one of the key factors involved in NETosis induction in a mouse model of acne skin inflammation. We found that NETosis was induced in P. acnes-induced skin inflammation in mice and that inhibition of NETosis ameliorated P. acnes-induced skin inflammation. In addition, our results demonstrated that inhibiting inflammasome activation could suppress NETosis induction in mouse skin. These results indicate that inflammasomes and NETosis can interact with each other to induce P. acnes-induced skin inflammation and suggest that targeting NETosis could be a potential treatment for inflammasome-mediated diseases as well as NETosis-related diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


