评估帕沙利西(一种选择性 PI3Kδ 抑制剂)对既往接受过治疗的 B 细胞恶性肿瘤患者的心脏安全性:CITADEL-101研究的结果
IF 2.9
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
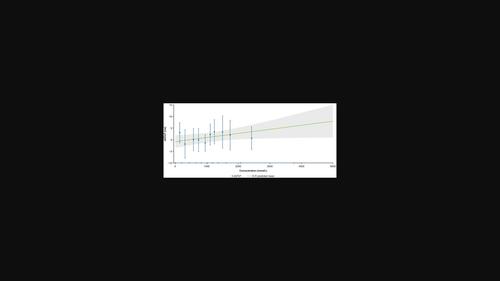
Parsaclisib是一种强效的选择性磷脂酰肌醇3激酶δ抑制剂,已被研究用于治疗B细胞恶性肿瘤,并在自身免疫性疾病和骨髓纤维化患者中进行了研究。CITADEL-101研究(NCT02018861)评估了帕沙利西在复发或难治性非霍奇金淋巴瘤患者中的安全性、耐受性和初步疗效。这项研究根据72例CITADEL-101研究入组患者的数据,评估了帕沙利西作为单药治疗的心脏安全性。69名患者接受了单药治疗,剂量分别为5、10、15、20、30和45毫克,每天一次,在指定时间收集了时间匹配的药代动力学和心电图测量数据。根据离群值分类分析,通过弗里德里西亚法(QTcF)校正心率(HR)后的QT间期、HR或心脏传导的离群值发生率未观察到剂量依赖性影响。根据中心倾向分析,各剂量组的ΔQTcF的最小平方均值(LSMs)(90%置信区间[CI])在-6.83(-18.8至5.19)至4.75毫秒(0.410至9.09)之间(低于20毫秒,大QT效应的阈值),不认为与剂量有关。此外,ΔHR、ΔPR 间期和ΔQRS 间期的 LSM 均很小。从浓度-ΔQTcF分析来看,所有剂量水平的预测ΔQTcF(90% CI)介于0.365(-1.75至2.48)和7.87毫秒(0.921至14.8)之间,CI的最高上限远低于20毫秒,因此,在调查的最高剂量水平(45毫克)之前,排除了较大的QT/QTc效应。总体而言,在所研究的剂量范围内,帕沙利西对QTcF的变化没有显示出浓度依赖性效应,对心率或心脏传导也没有显著影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Evaluation of the cardiac safety of parsaclisib, a selective PI3Kδ inhibitor, in patients with previously treated B-cell malignancies: Results from the CITADEL-101 study
Parsaclisib, a potent and selective phosphatidylinositol 3 kinase δ inhibitor, has been investigated for the treatment of B-cell malignancies and studied in patients with autoimmune diseases and myelofibrosis. The CITADEL-101 study (NCT02018861) assessed safety, tolerability, and preliminary efficacy of parsaclisib in patients with relapsed or refractory non-Hodgkin lymphoma. This study evaluated the cardiac safety of parsaclisib as monotherapy based on data from 72 patients enrolled in the CITADEL-101 study. Time-matched pharmacokinetic and ECG measurements were collected at specified times for 69 patients receiving monotherapy in doses of 5, 10, 15, 20, 30, and 45 mg once daily. Based on the categorical outlier analysis, no dose-dependent effect was observed on the incidence of outliers in QT interval corrected for heart rate (HR) by Fridericia's method (QTcF), HR, or cardiac conduction. Based on central tendency analysis, the least square means (LSMs) (90% confidence interval [CI]) of ΔQTcF from the central tendency analysis ranged from −6.83 (−18.8 to 5.19) to 4.75 ms (0.410–9.09) across dose groups (below 20 ms, the threshold of large QT effects) and was not considered dose dependent. Moreover, the LSMs of ΔHR, ΔPR interval, and ΔQRS interval were minor. From the concentration-ΔQTcF analyses, the predicted ΔQTcF (90% CI) for all dose levels was between 0.365 (−1.75 to 2.48) and 7.87 ms (0.921–14.8), with the highest upper limit of CIs well below 20 ms, and therefore, a large QT/QTc effect was ruled out up to the highest dose level (45 mg) investigated. Overall, parsaclisib at the dose ranges studied did not reveal concentration-dependent effects on change in QTcF and did not have a significant effect on HR or cardiac conduction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Pharmacology Research & Perspectives
Pharmacology, Toxicology and Pharmaceutics-General Pharmacology, Toxicology and Pharmaceutics
CiteScore
5.30
自引率
3.80%
发文量
120
审稿时长
20 weeks
期刊介绍:
PR&P is jointly published by the American Society for Pharmacology and Experimental Therapeutics (ASPET), the British Pharmacological Society (BPS), and Wiley. PR&P is a bi-monthly open access journal that publishes a range of article types, including: target validation (preclinical papers that show a hypothesis is incorrect or papers on drugs that have failed in early clinical development); drug discovery reviews (strategy, hypotheses, and data resulting in a successful therapeutic drug); frontiers in translational medicine (drug and target validation for an unmet therapeutic need); pharmacological hypotheses (reviews that are oriented to inform a novel hypothesis); and replication studies (work that refutes key findings [failed replication] and work that validates key findings). PR&P publishes papers submitted directly to the journal and those referred from the journals of ASPET and the BPS
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


