源自 CuMgAl-LDH 的 Cu2O/LDH 异质结:增强 Cu+ 在 CO2 电还原中的稳定性
IF 4.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
Cu+ 增强 C-C 偶联的独特能力使其在铜基催化剂上进行二氧化碳还原反应(CO2RR)领域具有重要意义,而 Cu+ 极易失活。在此,我们提出了一种简单可行的稳定策略,即通过添加抗坏血酸还原 Cu-Mg-Al 水滑石来稳定 Cu+ 物种。表征结果表明,卓越的稳定性得益于 Cu2O 和氢铝土的异质结构,其中保留的 Cu+ 物种来自 Cu2O,并减缓了 Cu+ 向 Cu0 的快速还原。本文章由计算机程序翻译,如有差异,请以英文原文为准。
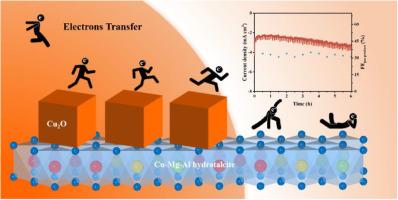
Cu2O/LDH heterojunction derived from CuMgAl-LDH: Enhanced stability of Cu+ in CO2 electroreduction
The unique ability of Cu+ to enhance C-C coupling makes it important in the field of CO2 reduction reaction (CO2RR) over Cu-based catalyst, while Cu+ is very prone to deactivation. Hereby, we propose a simple and feasible stabilization strategy to stabilize Cu+ species through reducing Cu-Mg-Al hydrotalcite by adding ascorbic acid, which owns stable product selectivity in a span of 6 h at −0.95 V vs.RHE. Characterization results show that the excellent stability is promoted by the heterostructure of Cu2O and hydrotalcite with retained Cu+ species, which derives from Cu2O and slow down the rapid reduction of Cu+ to Cu0.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Communications
化学-物理化学
CiteScore
6.20
自引率
2.70%
发文量
183
审稿时长
46 days
期刊介绍:
Catalysis Communications aims to provide rapid publication of significant, novel, and timely research results homogeneous, heterogeneous, and enzymatic catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


