印度基因治疗研究的最新进展
摘要
遗传性疾病具有渐进性,严重时会导致器官功能障碍或死亡。目前,95% 的遗传性疾病都没有永久性的治疗方案。不同的遗传方式、所涉及的基因类型以及基于人群的变异,使得为全球约 4 亿患者寻找合适的治疗方法变得更加复杂。基因疗法是治疗罕见遗传疾病的一种非常有前景的分子技术。基因治疗的原理是通过修复、替换、抑制以及最近的基因编辑来挽救疾病的表型。最新报告显示,与非病毒载体相比,越来越多的基因治疗临床试验使用病毒载体(64.2%)。腺相关病毒(AAV)和慢病毒等高效病毒载体系统的快速发展极大地推动了这一进展。值得注意的是,AAV 介导的基因疗法在遗传疾病治疗方面已显示出巨大潜力,这一点从最近针对眼部(NCT00999609)、血液(NCT00979238)和神经肌肉系统(NCT02122952)的临床试验中可见一斑。安全性和有效性是载体获得临床前和临床试验批准的两个最关键的特征。临床级载体的生产、评估和基因治疗产品的审批过程需要大量的技术开发、知识提升和巨额资金投入。此外,还需要训练有素的人才来满足不断进行技术创新的要求。这些因素共同导致每剂基因治疗产品的价格高昂,从而给基因治疗领域带来了挑战。印度次大陆在基因治疗临床试验方面历来落后于北美、欧洲、日本和其他国家,原因包括工业科学基础设施不足、缺乏可访问和有组织的患者数据库、资金投入少等。然而,在过去十年中,人们对罕见病的认识不断提高,Luxturna、Zolgensma、Hemgenix 等基因疗法在国际上获得批准,这也刺激了印度基因疗法的发展。鉴于这些进展,本文结合世界范围内的重大发展,概述了印度的基因治疗研究、监管流程和基因治疗临床试验的启动情况。我们简要介绍了印度各机构正在进行的基因治疗研究和新生的基因治疗产品制造。此外,我们还重点介绍了医疗界和患者为利用康复和基因治疗方案而采取的各种举措。我们简要讨论了印度基因治疗临床试验监管机构的作用和指导方针。我们希望这篇简明扼要的综述能凸显基因疗法为印度众多罕见病患者带来的希望。
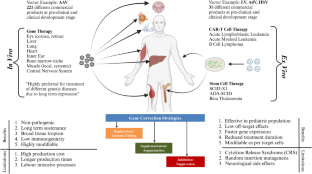
Inherited genetic disorders are progressive in nature and lead to organ dysfunction or death in severe cases. At present, there are no permanent treatment options for >95% of inherited disorders. Different modes of inheritance, type of gene(s) involved, and population-based variations add further complexity to finding suitable cures for approximately 400 million patients worldwide. Gene therapy is a very promising molecular technique for the treatment of rare genetic disorders. Gene therapy functions on the basis of restoration, replacement, inhibition, and, most recently, editing of gene(s) to rescue the disease phenotype. Recent reports show that increasing numbers of gene therapy clinical trials are using viral vectors (64.2%) when compared with non-viral vectors. Rapid development of efficient viral vector systems like the adeno-associated virus (AAV) and lentivirus has significantly contributed to this progress. Notably, AAV-mediated gene therapy has shown high potential for genetic disease treatment as evident from recent clinical trials for the eye (NCT00999609), blood (NCT00979238), and neuro-muscular systems (NCT02122952). Safety and efficacy are the two most critical features required for vector(s) to qualify for pre-clinical and clinical trial approval. The process of clinical-grade vector production, evaluation, and approvals for gene therapy products requires significant technological development, knowledge enhancement, and large financial investments. Additionally, trained manpower is required to meet the demands for constant technical innovation. These factors together contribute towards exorbitant prices for every dose of a gene therapy product and thus pose a challenge for the gene therapy field. The Indian subcontinent has traditionally lagged behind North America, Europe, Japan, and others in gene therapy clinical trials due to factors like inadequate industrial-scientific infrastructure, lack of accessible and organized patient databases, low financial investments, etc. However, over the last decade, increasing awareness of rare diseases, and international approvals of gene therapies such as Luxturna, Zolgensma, Hemgenix, etc., have spurred gene therapy development in India as well. In view of these advances, this article outlines gene therapy research, regulatory processes, and the launch of gene therapy clinical trials in India in the context of major developments worldwide. We briefly describe ongoing gene therapy research across Indian organizations and the nascent gene therapy product manufacturing. Further, we highlight the various initiatives from the medical and patient community to avail rehabilitation and gene therapy options. We briefly discuss the roles of regulatory agencies and guidelines for gene therapy clinical trials in India. We anticipate that this concise review will highlight the promise of gene therapy for the large population of rare disease patients in India.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


