完整、中间和空 AAV 包囊的分析表征。
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
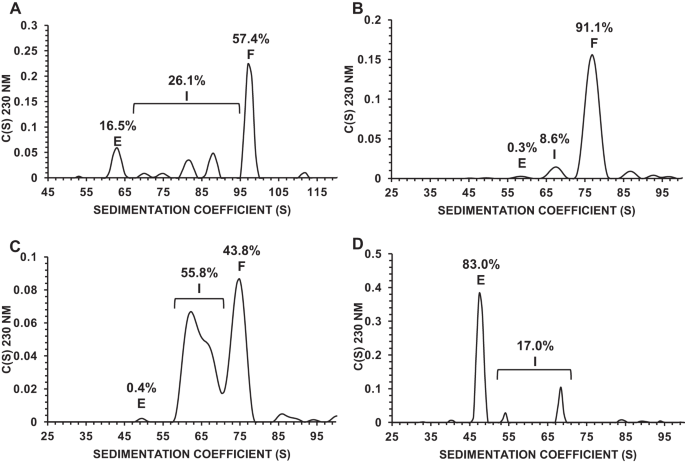
重组腺相关病毒(AAV)载体的生产过程会产生三种类型的囊壳:全囊壳、中间囊壳和空囊壳。虽然关于中间体和空壳对 AAV 产品安全性和功效的影响众说纷纭,但它们通常被认为是杂质,因为它们不是预期的完全完整的载体产品。这些杂质的存在可能会影响产品的功效,因为它们在细胞转导方面可能会与完全包装的 AAV 发生竞争,而且在给药过程中,由于囊壳负荷增加,可能会对患者的安全产生影响。为了确定中间体对药效的影响,我们将 AAV 制剂分离成富含完整、中间或空包囊的馏分。利用体外(感染性、基因表达、生物活性)和体内效价测定矩阵来确定效价与噬菌体含量的函数关系,我们的结果表明,虽然中间噬菌体有助于提高产品的载体基因组滴度,并且与全噬菌体具有同样的感染性,但它们并不影响 AAV 产品的效价。这项研究证实了减少和控制中间包囊水平对确保获得更有效的 AAV 产品至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Analytical characterization of full, intermediate, and empty AAV capsids
Manufacturing of recombinant adeno-associated virus (AAV) vectors produces three types of capsids: full, intermediate, and empty. While there are different opinions about the impact of intermediate and empty capsids on safety and efficacy of AAV products, they are generally considered impurities because they are not the intended fully intact vector product. The presence of these impurities could impact product efficacy due to potential competition with fully packaged AAVs for cellular transduction, as well as have potential implications to patient safety due to increased capsid load during dosing. To determine the impact of intermediate capsids on potency, an AAV preparation was separated into fractions enriched for full, intermediate, or empty capsids. Using a matrix of in vitro (infectivity, gene expression, biological activity) and in vivo potency assays to determine potency as a function of capsid content, our results indicate that while intermediate capsids contribute to the vector genome titer of the product and are equally as infectious as full capsids, they do not contribute to the potency of the AAV product. This study confirms the criticality of reducing and controlling the level of intermediate capsids to ensure a more efficacious AAV product.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


