采用壳聚糖支撑的 Ag 和 Ag2O 纳米簇催化 CO2 加氢制甲酸:量子半经验计算
IF 4.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究旨在考察壳聚糖支撑的 Ag 和 Ag2O 纳米粒子在还原 CO2 方面的潜力。利用裸弹带和半经验紧束缚计算系统地计算了还原反应的过渡态。研究发现,Ag 和 Ag2O 纳米团簇上的大电荷极化可以调节壳聚糖的表面反应活性。金属水合物的形成是还原 CO2 的决定性步骤。计算得出的活化能约为 1.5 eV,这表明 Ag 和 Ag2O / 壳聚糖可用作将甲酸转化为二氧化碳的催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
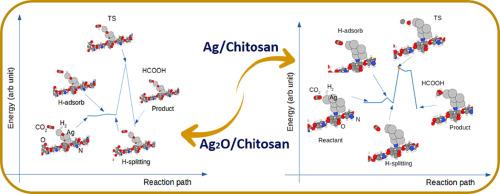
Adopting chitosan supported Ag and Ag2O nano-clusters for catalytic hydrogenation of CO2 to formic acid: A quantum semi-empirical calculation
This study's objective is to examine the potential of the chitosan-supported Ag and Ag2O nanoparticles on the reduction of CO2. The transition state of the reduction reaction was systematically calculated using the nudged elastic band and the semi-empirical tight-binding calculations. It is found that the large charge polarization on the Ag and Ag2O nanoclusters can modulate chitosan's surface reactivity. The formation of the metal hydrates is the rate-determining step for reducing CO2. The calculated activation energy of the order of 1.5 eV demonstrates that Ag and Ag2O /chitosan could be used as catalysts for converting to CO2 formic acid.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Communications
化学-物理化学
CiteScore
6.20
自引率
2.70%
发文量
183
审稿时长
46 days
期刊介绍:
Catalysis Communications aims to provide rapid publication of significant, novel, and timely research results homogeneous, heterogeneous, and enzymatic catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


