吡唑和吲唑氨基甲酸形成的理论研究
IF 2.1
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
使用 DFT 计算方法对吡唑和吲唑氨基甲酸的形成进行了理论研究。研究考虑了取代基和溶剂(使用显式和隐式溶剂模型)的影响。此外,还计算了氨基甲酸的去质子化及其对体系稳定性的影响。在中性体系中,与吡唑或吲唑与二氧化碳的非共价复合物相比,只有吲唑-1-氨基甲酸衍生物的形成是有利的。氨基甲酸的去质子化作用可高度稳定体系,防止其解离。本文章由计算机程序翻译,如有差异,请以英文原文为准。
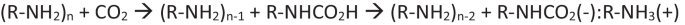
Theoretical study of the formation of pyrazole and indazole carbamic acids
A theoretical study of the formation of carbamic acids of pyrazole and indazole has been carried out using DFT computational methods. The effects of the substituents and the solvent (using explicit and implicit solvent models) have been considered. In addition, the deprotonation of the carbamic acid and its influence on the stability of the system has been calculated. In the neutral systems, only the formation of indazole-1-carbamic acid derivatives is favored vs. the non-covalent complexes between pyrazole or indazole with CO2. The deprotonation of the carbamic acid highly stabilizes the system preventing its dissociation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structural Chemistry
化学-化学综合
CiteScore
3.80
自引率
11.80%
发文量
227
审稿时长
3.7 months
期刊介绍:
Structural Chemistry is an international forum for the publication of peer-reviewed original research papers that cover the condensed and gaseous states of matter and involve numerous techniques for the determination of structure and energetics, their results, and the conclusions derived from these studies. The journal overcomes the unnatural separation in the current literature among the areas of structure determination, energetics, and applications, as well as builds a bridge to other chemical disciplines. Ist comprehensive coverage encompasses broad discussion of results, observation of relationships among various properties, and the description and application of structure and energy information in all domains of chemistry.
We welcome the broadest range of accounts of research in structural chemistry involving the discussion of methodologies and structures,experimental, theoretical, and computational, and their combinations. We encourage discussions of structural information collected for their chemicaland biological significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


