Aritra Bhattacharyya, Ranjha Khan, Joanna Y Lee, Gizachew Tassew, Babak Oskouian, Maria L Allende, Richard L Proia, Xiaoyang Yin, Javier G Ortega, Mallar Bhattacharya, Julie D Saba
下载PDF
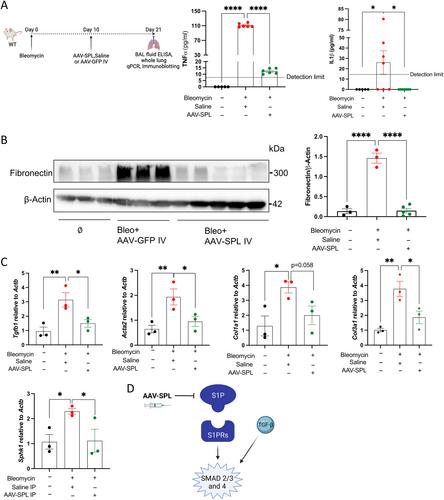
{"title":"在肺纤维化动物模型中使用 AAV9-SGPL1 进行基因治疗。","authors":"Aritra Bhattacharyya, Ranjha Khan, Joanna Y Lee, Gizachew Tassew, Babak Oskouian, Maria L Allende, Richard L Proia, Xiaoyang Yin, Javier G Ortega, Mallar Bhattacharya, Julie D Saba","doi":"10.1002/path.6256","DOIUrl":null,"url":null,"abstract":"<p>Idiopathic pulmonary fibrosis (IPF) is a progressive scarring disease of the lung that leads rapidly to respiratory failure. Novel approaches to treatment are urgently needed. The bioactive lipid sphingosine-1-phosphate (S1P) is increased in IPF lungs and promotes proinflammatory and profibrotic TGF-β signaling. Hence, decreasing lung S1P represents a potential therapeutic strategy for IPF. S1P is degraded by the intracellular enzyme S1P lyase (SPL). Here we find that a knock-in mouse with a missense SPL mutation mimicking human disease resulted in reduced SPL activity, increased S1P, increased TGF-β signaling, increased lung fibrosis, and higher mortality after injury compared to wild type (WT). We then tested adeno-associated virus 9 (AAV9)-mediated overexpression of human <i>SGPL1</i> (AAV-SPL) in mice as a therapeutic modality. Intravenous treatment with AAV-SPL augmented lung SPL activity, attenuated S1P levels within the lungs, and decreased injury-induced fibrosis compared to controls treated with saline or only AAV. We confirmed that AAV-SPL treatment led to higher expression of SPL in the epithelial and fibroblast compartments during bleomycin-induced lung injury. Additionally, AAV-SPL decreased expression of the profibrotic cytokines TNFα and IL1β as well as markers of fibroblast activation, such as fibronectin (<i>Fn1</i>), <i>Tgfb1</i>, <i>Acta2</i>, and collagen genes in the lung. Taken together, our results provide proof of concept for the use of AAV-SPL as a therapeutic strategy for the treatment of IPF. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"263 1","pages":"22-31"},"PeriodicalIF":5.6000,"publicationDate":"2024-02-09","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6256","citationCount":"0","resultStr":"{\"title\":\"Gene therapy with AAV9-SGPL1 in an animal model of lung fibrosis\",\"authors\":\"Aritra Bhattacharyya, Ranjha Khan, Joanna Y Lee, Gizachew Tassew, Babak Oskouian, Maria L Allende, Richard L Proia, Xiaoyang Yin, Javier G Ortega, Mallar Bhattacharya, Julie D Saba\",\"doi\":\"10.1002/path.6256\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p>Idiopathic pulmonary fibrosis (IPF) is a progressive scarring disease of the lung that leads rapidly to respiratory failure. Novel approaches to treatment are urgently needed. The bioactive lipid sphingosine-1-phosphate (S1P) is increased in IPF lungs and promotes proinflammatory and profibrotic TGF-β signaling. Hence, decreasing lung S1P represents a potential therapeutic strategy for IPF. S1P is degraded by the intracellular enzyme S1P lyase (SPL). Here we find that a knock-in mouse with a missense SPL mutation mimicking human disease resulted in reduced SPL activity, increased S1P, increased TGF-β signaling, increased lung fibrosis, and higher mortality after injury compared to wild type (WT). We then tested adeno-associated virus 9 (AAV9)-mediated overexpression of human <i>SGPL1</i> (AAV-SPL) in mice as a therapeutic modality. Intravenous treatment with AAV-SPL augmented lung SPL activity, attenuated S1P levels within the lungs, and decreased injury-induced fibrosis compared to controls treated with saline or only AAV. We confirmed that AAV-SPL treatment led to higher expression of SPL in the epithelial and fibroblast compartments during bleomycin-induced lung injury. Additionally, AAV-SPL decreased expression of the profibrotic cytokines TNFα and IL1β as well as markers of fibroblast activation, such as fibronectin (<i>Fn1</i>), <i>Tgfb1</i>, <i>Acta2</i>, and collagen genes in the lung. Taken together, our results provide proof of concept for the use of AAV-SPL as a therapeutic strategy for the treatment of IPF. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>\",\"PeriodicalId\":232,\"journal\":{\"name\":\"The Journal of Pathology\",\"volume\":\"263 1\",\"pages\":\"22-31\"},\"PeriodicalIF\":5.6000,\"publicationDate\":\"2024-02-09\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6256\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"The Journal of Pathology\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://onlinelibrary.wiley.com/doi/10.1002/path.6256\",\"RegionNum\":2,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"ONCOLOGY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6256","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


