基因疗法可纠正硅烷基糖苷酸沉积症小鼠的神经系统缺陷。
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
由于神经氨酸酶 1(NEU1)基因突变,I 型硅脂质沉着症(粘脂质沉着症 I 型)患者通常表现为肌阵挛、癫痫发作、共济失调、樱桃红色斑点和失明。目前,还没有治疗sialidosis的方法。在这项研究中,我们开发了一种腺相关病毒(AAV)介导的基因疗法,用于神经氨酸酶1基因敲除(Neu1-/-)的小鼠硅质酸沉着症模型。载体 AAV9-P3-NP 包括人 NEU1 启动子、NEU1 cDNA、IRES 和 CTSA cDNA。未经处理的Neu1-/-小鼠神经系统(包括大脑、脊髓和背根神经节)中出现星形胶质细胞增多和小胶质细胞LAMP1聚集,同时运动功能受损。通过脑室内注射在新生 Neu1-/- 小鼠体内共表达 NEU1 和保护性蛋白/胰蛋白酶 A(PPCA),并通过面部静脉注射降低其效果。面静脉注射还能提高 Neu1-/- 小鼠的握力和存活率。因此,脑脊液输送AAV9-P3-NP可纠正sialidosis小鼠的神经功能缺陷,是治疗sialidosis I型患者的一种合适方法。然后,受 PPCA 保护的 NEU1 被送往溶酶体,β-Gal 与该复合物结合形成多酶复合物,以执行其功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
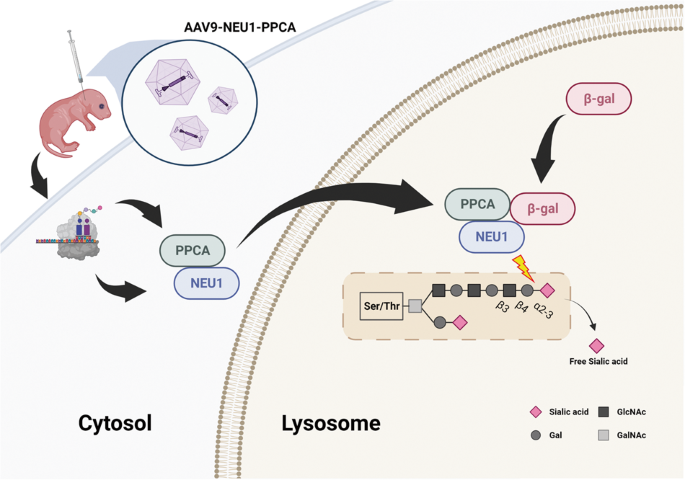
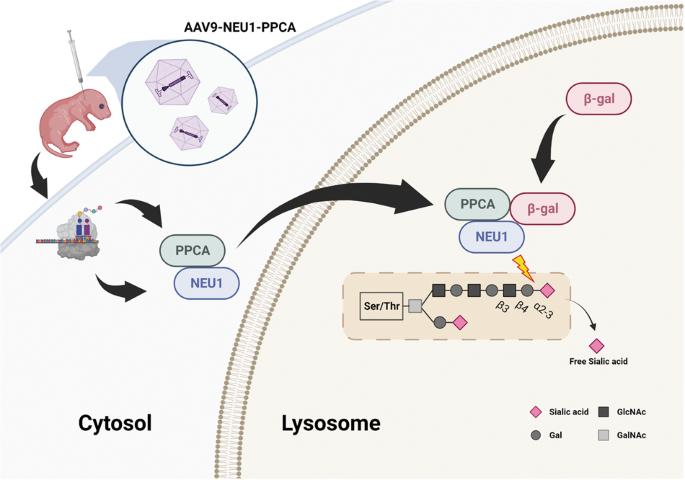
Gene therapy corrects the neurological deficits of mice with sialidosis
Patients with sialidosis (mucolipidosis type I) type I typically present with myoclonus, seizures, ataxia, cherry-red spots, and blindness because of mutations in the neuraminidase 1 (NEU1) gene. Currently, there is no treatment for sialidosis. In this study, we developed an adeno-associated virus (AAV)-mediated gene therapy for a Neu1 knockout (Neu1−/−) mouse model of sialidosis. The vector, AAV9-P3-NP, included the human NEU1 promoter, NEU1 cDNA, IRES, and CTSA cDNA. Untreated Neu1−/− mice showed astrogliosis and microglial LAMP1 accumulation in the nervous system, including brain, spinal cord, and dorsal root ganglion, together with impaired motor function. Coexpression of NEU1 and protective protein/cathepsin A (PPCA) in neonatal Neu1−/− mice by intracerebroventricular injection, and less effective by facial vein injection, decreased astrogliosis and LAMP1 accumulation in the nervous system and improved rotarod performance of the treated mice. Facial vein injection also improved the grip strength and survival of Neu1−/− mice. Therefore, cerebrospinal fluid delivery of AAV9-P3-NP, which corrects the neurological deficits of mice with sialidosis, could be a suitable treatment for patients with sialidosis type I.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


