5Z,9Z-Dienoic Acid 的碘δ-内酯体外抗肿瘤活性研究
IF 1
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
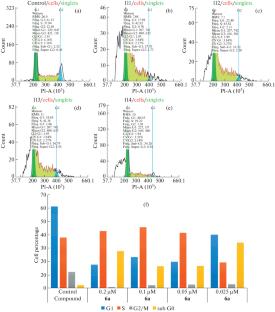
摘要利用钛催化的芳香族 1,2-二烯与含 O-异烯的分子间交叉环镁化反应,在关键阶段合成了 11-苯基十一碳-5Z,9Z-二烯酸衍生的苯基取代碘-δ-内酯,收率为 94%。研究了合成的 5Z,9Z-二烯酸烷基和苯基取代碘-δ-内酯对 Jurkat、K562、U937、HL60 和 Hek293 细胞株的体外细胞毒性活性,并使用流式细胞仪检测了它们对细胞周期的影响和诱导细胞凋亡的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Study of the Antitumor Activity in Vitro of Iodo-δ-Lactones of 5Z,9Z-Dienoic Acid
Previously unknown phenyl-substituted iodo-δ-lactone derived from 11‑phenylundeca-5Z,9Z-dienoic acid was synthesized in 94% yield using the Ti-catalyzed intermolecular cross-cyclomagnesiation of aromatic 1,2-diene with O‑containing allene at the key stage. The in vitro cytotoxic activity of synthesized alkyl- and phenyl-substituted iodo-δ-lactones of 5Z,9Z-dienoic acids against the Jurkat, K562, U937, HL60, and Hek293 cell lines was studied, and their effect on the cell cycle and the ability to induce apoptosis were investigated using flow cytometry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Doklady Chemistry
化学-化学综合
CiteScore
1.20
自引率
12.50%
发文量
7
审稿时长
6-12 weeks
期刊介绍:
Doklady Chemistry is a journal that publishes new research in chemistry and chemical engineering of great significance. Initially the journal was a forum of the Russian Academy of Science and published only best contributions from Russia in the form of short articles. Now the journal welcomes submissions from any country in the English or Russian language. Every manuscript must be recommended by Russian or foreign members of the Russian Academy of Sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


