α、γ-杂交肽中螺旋-翻转-螺旋型图案的晶体结构分析
IF 2.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
利用短合成肽序列模拟蛋白质超二级结构在合成蛋白质设计、催化和药物发现领域具有重要意义。在这项研究中,我们展示了一系列由短α,γ-杂交肽衍生而来的螺旋-转-螺旋图案,其中包含中心位置的 E-αβ- 不饱和γ-氨基酸。通过改变中心残基上反式双键的数量,可以调整螺旋的位置。将合成的七残基螺旋-翻转-螺旋图案与天然的钙结合螺旋-翻转-螺旋图案叠加,揭示了在短肽序列中设计三维螺旋-翻转-螺旋图案的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
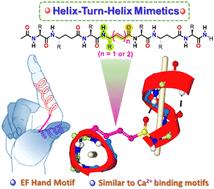
Crystal structure analysis of helix–turn–helix type motifs in α,γ-hybrid peptides†
Mimicking protein supersecondary structures using short synthetic peptide sequences holds significant importance in the fields of synthetic protein design, catalysis, and drug discovery. In this study, we present a series of helix–turn–helix motifs derived from short α,γ-hybrid peptides, incorporating centrally positioned E-α,β-unsaturated γ-amino acids. By varying the number of trans double bonds at the central residue, the positioning of the helices can be adjusted. Superimposing the synthetic seven-residue helix–turn–helix motif with the natural calcium-binding helix–turn–helix motif revealed the potential to design three-dimensional helix–turn–helix motifs within short peptide sequences.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

CrystEngComm
化学-化学综合
CiteScore
5.50
自引率
9.70%
发文量
747
审稿时长
1.7 months
期刊介绍:
Design and understanding of solid-state and crystalline materials
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


