设计和验证研究问卷--第二部分。
Q2 Medicine
Perspectives in Clinical Research
Pub Date : 2024-01-01
Epub Date: 2024-01-09
DOI:10.4103/picr.picr_318_23
引用次数: 0
摘要
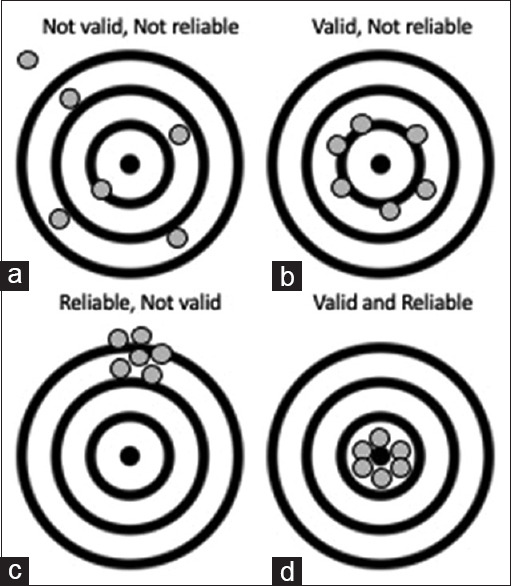
有效性和可靠性是指研究工具的准确性和一致性。在本系列的上一篇文章中,我们探讨了研究问卷的编制。在本文中,我们将讨论确定研究问卷有效性和可靠性的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Designing and validating a research questionnaire - Part 2.
Validity and reliability refer to the accuracy and consistency of a research tool. In the previous article in this series, we examined the development of a research questionnaire. In this article, we discuss the methods of determining the validity and reliability of a research questionnaire.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Perspectives in Clinical Research
Medicine-Medicine (all)
CiteScore
2.90
自引率
0.00%
发文量
41
审稿时长
36 weeks
期刊介绍:
This peer review quarterly journal is positioned to build a learning clinical research community in India. This scientific journal will have a broad coverage of topics across clinical research disciplines including clinical research methodology, research ethics, clinical data management, training, data management, biostatistics, regulatory and will include original articles, reviews, news and views, perspectives, and other interesting sections. PICR will offer all clinical research stakeholders in India – academicians, ethics committees, regulators, and industry professionals -a forum for exchange of ideas, information and opinions.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


