用于锂金属电池的单相局部高浓度固体聚合物电解质
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
固体聚合物是很有前途的锂金属电池电解质,但它们也有局限性:它们无法同时实现高离子电导率、良好的机械强度以及与高电压阴极的兼容性,同时抑制锂枝晶。在此,我们设计了一类基于聚合物混合物的局部高浓度固体聚合物电解质,这种电解质被称为 "锂聚合物 F 稀释剂(LPIFD)"。锂聚合物(盐中聚合物)可确保连续的锂离子传导通道,并有助于形成固体电解质间相(SEI),而F稀释剂(惰性含氟聚合物)则可增加机械强度。研究表明,基于混溶聚合物混合物的单相 LPIFD 缺乏相界,可形成无有机物且富含锂氟的 SEI,从而有效抑制锂枝晶。单相 LPIFD 的离子电导率为 3.0 × 10-4 S cm-1,可使锂阳极达到 99.1% 的高库仑效率和 3.7 mA cm-2 的临界电流密度。此外,由于能够形成富含 F 的阴极电解质间相,镍钴锰锂电池在 4.5 V 高工作电压下的循环寿命可达 450 次。这一设计将推动高能锂金属电池聚合物电解质的商业化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
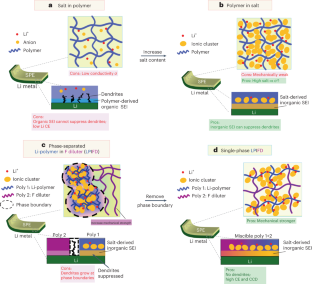
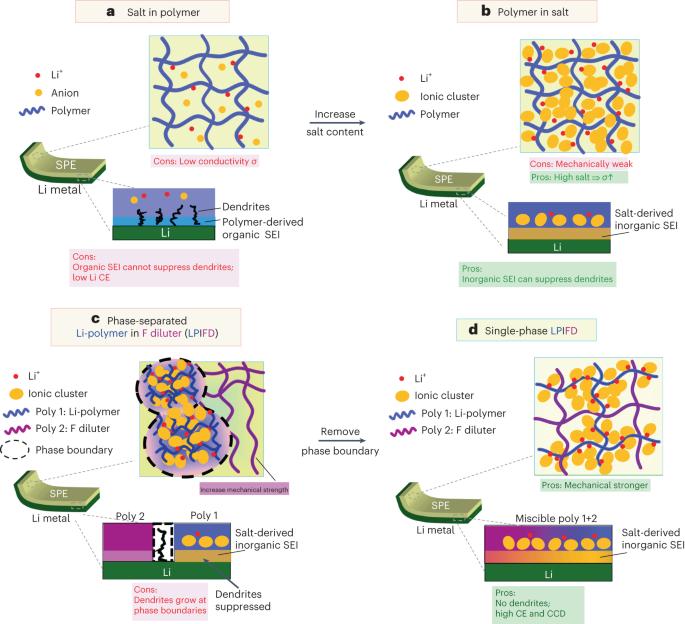
Single-phase local-high-concentration solid polymer electrolytes for lithium-metal batteries
Solid polymers are promising electrolytes for Li-metal batteries, but they have limitations: they cannot simultaneously achieve high ionic conductivity, good mechanical strength and compatibility with high-voltage cathodes while suppressing Li dendrites. Here, we design a class of locally high-concentration solid polymer electrolytes based on polymer blends, which are termed Li-polymer in F diluter (LPIFD). The Li-polymer (polymer-in-salt) ensures continuous Li-ion conduction channels and contributes to the solid electrolyte interphase (SEI), and the F diluter (inert fluorinated polymer) adds mechanical strength. Studies reveal that a single-phase LPIFD, which is based on a miscible polymer blend, lacks phase boundaries and forms an organic-less and LiF-rich SEI, effectively suppressing lithium dendrites. The single-phase LPIFD delivers ionic conductivity of 3.0 × 10−4 S cm−1, and enables the Li anode to reach a high coulombic efficiency of 99.1% and a critical current density of 3.7 mA cm−2. Furthermore, the ability to form an F-rich cathode electrolyte interphase allows LiNi0.8Co0.1Mn0.1O2||Li cells to achieve a cycle life of 450 cycles at a high operating voltage of 4.5 V. This design will inspire efforts to commercialize polymer electrolytes for high-energy Li-metal batteries. Batteries with solid polymer electrolytes face challenges in electrochemical stability and compatibility with high-voltage cathodes. Chunsheng Wang and colleagues have developed a polymer blend with a high Li salt concentration that enhances the stability of solid polymer electrolytes and achieves promising electrochemical performance in full-cell applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


