从羟肟酸合成 1,4,2-二恶唑的新途径
IF 1
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
提出了一种从乙烯基芳基醚和羟肟酸获得功能取代的 1,4,2-二恶唑的新合成方法。目标杂环是在温和的条件下以良好的产率形成的。研究表明,闭环是通过形成作为中间体的羟基(甲基)芳基羟肟酸酯来实现的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
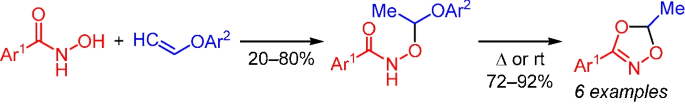
A new route for the synthesis of 1,4,2-dioxazoles from hydroxamic acids
A new synthetic method to obtain functionally substituted 1,4,2-dioxazoles from vinyl aryl ethers and hydroxamic acids was proposed. The target heterocycles were formed under mild conditions in good yields. It was shown that ring closure occurs via the formation of aroxy(methyl) arylhydroxamates as intermediates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
2.90
自引率
13.30%
发文量
98
审稿时长
1 months
期刊介绍:
The international journal Chemistry of Heterocyclic Compounds publishes original papers, short communications, reviews, and mini-reviews dealing with problems in the field of heterocyclic chemistry in Russian and English. The Journal also publishes reviews and annotations on new books and brief reports on conferences in the field of heterocyclic chemistry, as well as commemoratives dedicated to prominent heterocyclic chemists.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


