CSTB基因置换可改善小鼠1型进行性肌阵挛癫痫的神经炎症、神经变性和共济失调。
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
EPM1 是最常见的进行性肌阵挛癫痫(Progressive Myoclonus Epilepsy),其特征是儿童晚期发病,肌阵挛、癫痫发作、共济失调、精神疾病不断恶化,并缩短寿命。EPM1 的病因是 CSTB(胱抑素 B)启动子中十二聚体重复序列的扩增,这种扩增会显著降低但不会消除基因的表达。EPM1 的发病时间相对较晚,而且始终存在极少量的蛋白质产物,因此是基因替代疗法的一个有利靶点。如果及早治疗,这些儿童发育正常的大脑就能从随之而来的神经退行性变中解救出来,而且他们的交叉反应免疫物质(CRIM)阳性状态也能大大降低与转基因相关的毒性。我们在 Cstb 基因敲除小鼠中进行了一项概念验证 CSTB 基因替代研究,将 CBh 启动子驱动的全长人 CSTB 包装在 AAV9 中,并在出生后第 21 天和第 60 天给药。小鼠分别在 2 个月或 9 个月大时被处死。我们观察到神经炎症通路基因和小脑颗粒细胞层凋亡的表达水平有了明显改善,运动障碍也有所改善。这些数据表明,基因替代是治疗EPM1的一种很有前景的方法,可使患儿和家庭免受这种严重神经退行性疾病的摧残。本文章由计算机程序翻译,如有差异,请以英文原文为准。
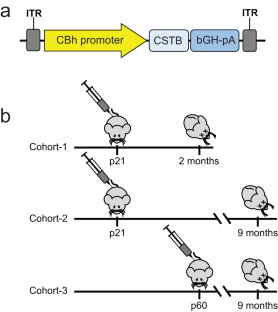
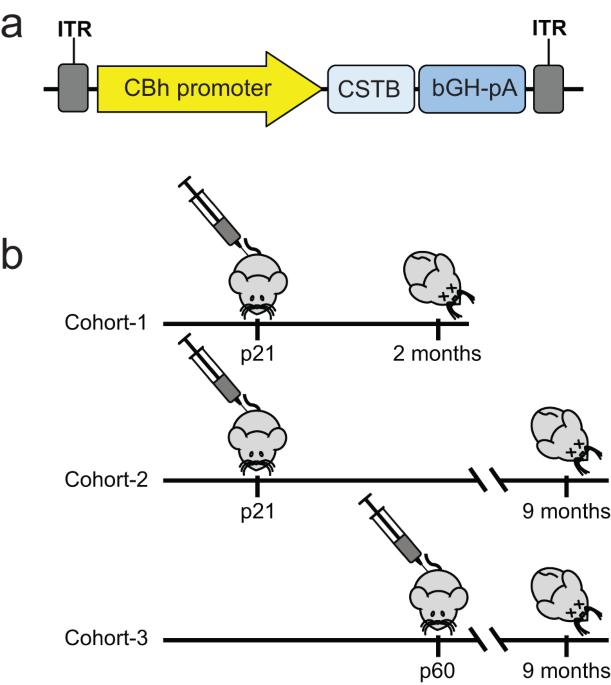
CSTB gene replacement improves neuroinflammation, neurodegeneration and ataxia in murine type 1 progressive myoclonus epilepsy
EPM1 is the most common form of Progressive Myoclonus Epilepsy characterized by late-childhood onset, ever-worsening and disabling myoclonus, seizures, ataxia, psychiatric disease, and shortened lifespan. EPM1 is caused by expansions of a dodecamer repeat sequence in the promoter of CSTB (cystatin B), which dramatically reduces, but does not eliminate, gene expression. The relatively late onset and consistent presence of a minimal amount of protein product makes EPM1 a favorable target for gene replacement therapy. If treated early, these children’s normally developed brains could be rescued from the neurodegeneration that otherwise follows, and their cross-reactive immunological material (CRIM) positive status greatly reduces transgene related toxicity. We performed a proof-of-concept CSTB gene replacement study in Cstb knockout mice by introducing full-length human CSTB driven by the CBh promoter packaged in AAV9 and administered at postnatal days 21 and 60. Mice were sacrificed at 2 or 9 months of age, respectively. We observed significant improvements in expression levels of neuroinflammatory pathway genes and cerebellar granule cell layer apoptosis, as well as amelioration of motor impairment. The data suggest that gene replacement is a promising therapeutic modality for EPM1 and could spare affected children and families the ravages of this otherwise severe neurodegenerative disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


