高含量多模式分析支持将 IL-7/IL-7 受体轴作为原发性斯约格伦综合征的相关治疗靶点
IF 7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
虽然 IL-7/IL-7R 轴参与 pSS 与 T 细胞有关,但人们对该通路与其他免疫细胞的关系及其在自身免疫中的作用知之甚少。利用高内涵多组学数据,我们旨在确定表达 IL-7R 的细胞的特征,以及 IL-7/IL-7R 通路在 pSS 病理生理学中的参与情况。结果我们发现血液中的 4 个基因 IL-7 模块(IKZF4、KIAA0040、PGAP1 和 SOS1)与患者的抗 SSA/Ro 阳性和疾病活动性相关,组织中的 5 个基因 IL-7 模块(IL7R、PCED1B、TNFSF8、ADAM19 和 MYBL1)与浸润严重程度相关。我们证实了 IL-7R 在 T 细胞亚群中的表达,并进一步观察到 IL-7R 在双阴性(DN)B 细胞,尤其是 DN2 B 细胞中的上调。与巩膜炎患者相比,IL-7R 在巩膜炎患者中的表达增加,并根据浸润程度而有所变化。当 IL-7R 表达时,主要存在于上皮细胞、CD4+和 CD8+ T 细胞、切换记忆 B 细胞、DN B 细胞和 M1 巨噬细胞中。利用这两个基因模块可以指导精准医疗中抗IL-7R靶向疗法的实施。本文章由计算机程序翻译,如有差异,请以英文原文为准。
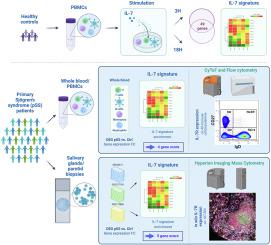

High-content multimodal analysis supports the IL-7/IL-7 receptor axis as a relevant therapeutic target in primary Sjögren's syndrome
Objective
While the involvement of IL-7/IL-7R axis in pSS has been described in relation to T cells, little is known about the contribution of this pathway in relationship with other immune cells, and its implication in autoimmunity. Using high-content multiomics data, we aimed at characterizing IL-7R expressing cells and the involvement of IL-7/IL-7R pathway in pSS pathophysiology.
Methods
An IL-7 signature established using RNA-sequencing of human PBMCs incubated with IL-7 was applied to 304 pSS patients, and on RNA-Seq datasets from tissue biopsies. High-content immunophenotyping using flow and imaging mass cytometry was developed to characterize peripheral and in situ IL-7R expression.
Results
We identified a blood 4-gene IL-7 module (IKZF4, KIAA0040, PGAP1 and SOS1) associated with anti-SSA/Ro positiveness in patients as well as disease activity, and a tissue 5-gene IL-7 module (IL7R, PCED1B, TNFSF8, ADAM19, MYBL1) associated with infiltration severity. We confirmed expression of IL-7R on T cells subsets, and further observed upregulation of IL-7R on double-negative (DN) B cells, and especially DN2 B cells. IL-7R expression was increased in pSS compared to sicca patients with variations seen according to the degree of infiltration. When expressed, IL-7R was mainly found on epithelial cells, CD4+ and CD8+ T cells, switched memory B cells, DN B cells and M1 macrophages.
Conclusion
This exhaustive characterization of the IL-7/IL-7R pathway in pSS pathophysiology established that two IL-7 gene modules discriminate pSS patients with a high IL-7 axis involvement. Their use could guide the implementation of an anti-IL-7R targeted therapy in a precision medicine approach.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of autoimmunity
医学-免疫学
CiteScore
27.90
自引率
1.60%
发文量
117
审稿时长
17 days
期刊介绍:
The Journal of Autoimmunity serves as the primary publication for research on various facets of autoimmunity. These include topics such as the mechanism of self-recognition, regulation of autoimmune responses, experimental autoimmune diseases, diagnostic tests for autoantibodies, as well as the epidemiology, pathophysiology, and treatment of autoimmune diseases. While the journal covers a wide range of subjects, it emphasizes papers exploring the genetic, molecular biology, and cellular aspects of the field.
The Journal of Translational Autoimmunity, on the other hand, is a subsidiary journal of the Journal of Autoimmunity. It focuses specifically on translating scientific discoveries in autoimmunity into clinical applications and practical solutions. By highlighting research that bridges the gap between basic science and clinical practice, the Journal of Translational Autoimmunity aims to advance the understanding and treatment of autoimmune diseases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


