不同成分浓度下 PEGDA/Irgacure 水溶液液滴中的聚合情况
摘要
摘要实验研究了PEGDA/Irgacure 2959水溶液在紫外辐射下发生聚合前后的蒸发行为、液相对流行为和自由表面温度。在聚合物基的初始浓度\(C_{0}\)范围内,得到了液滴自由表面的温度数据。因为\(C_{0}= 35\)% and less, no polymerization area was observed (according to thermal images). The temperature gradients observed over the droplet surface were very low (less than 0.3°C). At \(C_{0}= 65\)% and \(C_{0}= 90\)%, the average temperature \(T_{\rm S}\) on the free surface of droplet changed during polymerization by 2.8C° and 3.7°C, respectively. After polymerization, \(T_{\rm S}\) for the hydrogel became higher than \(T_{\rm S }\) of droplet of pure water. The average velocity of motion of polyamide particles \(U_{C}\) for water was 2.2–2.4 times higher than that for the PEGDA solution with the initial concentration \(C_{0} = 65\)%, which is associated with the higher viscosity of the solution. At the onset of polymerization, the velocity \(U_{C}\)dropped to 0 mm/s in a very short time. With increase in the concentration \(C_{0}\) in the pre-polymer, the time of polymerization reaction start decreased significantly, which shortened the time in which the particles stopped completely. Different limiting factors govern evaporation of a water droplet and a hydrogel droplet. The evaporation rate of droplet of hydrogel decreased with time due to the porous structure of the hydrogel.
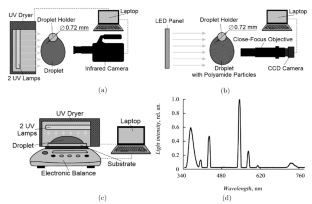
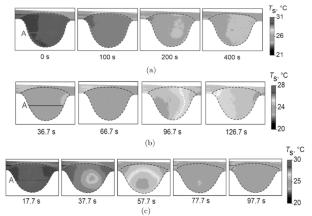
The authors experimentally studied the behavior of evaporation, convection in the liquid, and temperature of the free surface of a pendant droplet of aqueous solution of PEGDA/Irgacure 2959 before and after the onset of polymerization under UV radiation. Data on the temperature of the free surface of droplet were obtained for a wide range of the initial concentration of the polymer base \(C_{0}\). For \(C_{0}= 35\)% and less, no polymerization area was observed (according to thermal images). The temperature gradients observed over the droplet surface were very low (less than 0.3°C). At \(C_{0}= 65\)% and \(C_{0}= 90\)%, the average temperature \(T_{\rm S}\) on the free surface of droplet changed during polymerization by 2.8C° and 3.7°C, respectively. After polymerization, \(T_{\rm S}\) for the hydrogel became higher than \(T_{\rm S }\) of droplet of pure water. The average velocity of motion of polyamide particles \(U_{C}\) for water was 2.2–2.4 times higher than that for the PEGDA solution with the initial concentration \(C_{0} = 65\)%, which is associated with the higher viscosity of the solution. At the onset of polymerization, the velocity \(U_{C}\)dropped to 0 mm/s in a very short time. With increase in the concentration \(C_{0}\) in the pre-polymer, the time of polymerization reaction start decreased significantly, which shortened the time in which the particles stopped completely. Different limiting factors govern evaporation of a water droplet and a hydrogel droplet. The evaporation rate of droplet of hydrogel decreased with time due to the porous structure of the hydrogel.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


