育宫汤联合质子泵抑制剂治疗难治性胃食管反流病的临床疗效:随机、双盲、双剂临床试验研究方案
IF 3.4
2区 医学
Q1 Medicine
引用次数: 0
摘要
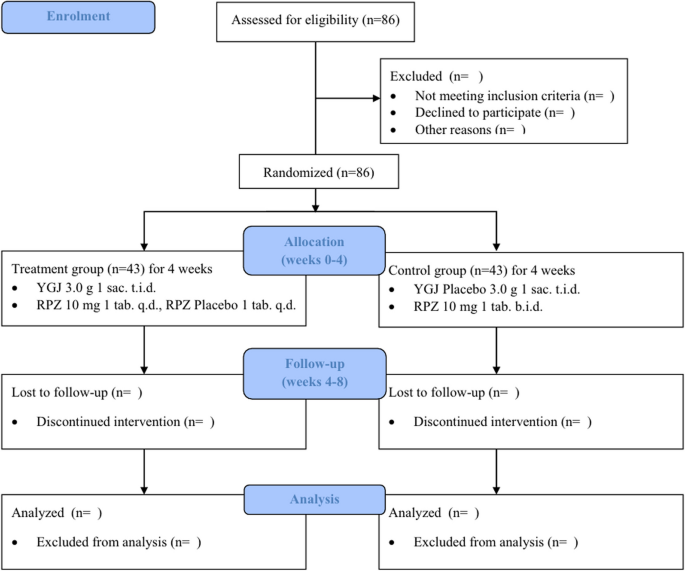
育胃汤(YGJ)是一种用于治疗胃食管反流病(GERD)症状的草药处方。虽然针对胃食管反流病进行了许多临床前和临床研究,但缺乏盲法研究的证据来排除安慰剂效应。因此,本方案提出了一项单中心、随机、双盲、双假的临床试验,以客观评估 YGJ 和雷贝拉唑(RPZ)联合用药对曾接受质子泵抑制剂(PPIs)治疗但仍有症状的胃食管反流病患者的疗效和安全性。共有 86 名难治性胃食管反流病患者(rGERD)将按 1:1 的比例随机分配到治疗组(YGJ 和雷贝拉唑(10 毫克/天))和对照组(双剂量雷贝拉唑(20 毫克/天)),治疗 4 周(第 0-4 周),然后随访 4 周(第 4-8 周)。主要终点将分析胃食管反流症状频率量表。反流病问卷、反流症状评分、胃食管反流病健康相关生活质量、总体治疗评价、脾气虚问卷、达穆姆问卷和消化不良视觉模拟量表将用于评估治疗对胃食管反流病相关症状和生活质量的影响,并比较不同亚组的治疗效果。安全性测试将通过调查不良事件进行分析。该临床试验将是首个严格的双盲、双假、安慰剂对照研究,以精确评估 YGJ 和 PPIs 联合治疗胃食管反流病的疗效和安全性。这项研究的结果将为rGERD患者选择植物药治疗提供可靠的临床依据。临床研究信息服务(注册号:KCT0008600,2023年7月13日,https://cris.nih.go.kr )。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Clinical efficacy of Yukgunja-tang combined with a proton pump inhibitor for refractory gastroesophageal reflux disease: study protocol for randomized, double-blind, double-dummy clinical trial
Yukgunja-tang (YGJ) is an herbal prescription used to treat the symptoms of gastroesophageal reflux disease (GERD). Although many preclinical and clinical studies on YGJ have been conducted on GERD, there is a lack of evidence from blinded studies to exclude placebo effects. Therefore, this protocol proposes a clinical trial that is single-centered, randomized, double-blinded, double-dummy to objectively evaluate the efficacy and safety of co-administered YGJ and rabeprazole (RPZ) in patients with GERD previously treated with proton pump inhibitors (PPIs) and still experiencing symptoms. A total of 86 participants with refractory GERD (rGERD) will be randomized in a 1:1 ratio to the treatment [YGJ and RPZ (10 mg/d)] and control groups [double-dose RPZ (20 mg/d)] for 4 weeks of treatment (weeks 0–4) followed by 4 weeks of follow-up (weeks 4–8). The Frequency Scale for the Symptoms of GERD will be analyzed for the primary endpoint. Reflux Disease Questionnaire, Reflux Symptom Score, GERD-Health Related Quality of Life, Overall Treatment Evaluation, Spleen Qi Deficiency Questionnaire, Damum Questionnaire, and dyspepsia Visual Analogue Scale will be used to evaluate treatment effects on GERD related symptoms and quality of life and to compare treatment effects by subgroups. Safety tests will be analyzed by investigating adverse events. This clinical trial will be the first rigorous double-blind, double-dummy, placebo-controlled study to precisely evaluate the efficacy and safety of the combination of YGJ and PPIs in the treatment of rGERD. The results of this study will provide a reliable clinical basis for selecting botanical drug treatments for patients with rGERD. Clinical Research Information Service (registration number: KCT0008600, July 13, 2023, https://cris.nih.go.kr ).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

BMC Complementary and Alternative Medicine
INTEGRATIVE & COMPLEMENTARY MEDICINE-
CiteScore
7.00
自引率
0.00%
发文量
0
审稿时长
3 months
期刊介绍:
BMC Complementary Medicine and Therapies is an open access journal publishing original peer-reviewed research articles on interventions and resources that complement or replace conventional therapies, with a specific emphasis on research that explores the biological mechanisms of action, as well as their efficacy, safety, costs, patterns of use and/or implementation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


