通过分析评估阈值筛选研究不同批次非聚氯乙烯软输液袋中的可萃取物和可浸出物
IF 3.7
4区 工程技术
Q2 ENGINEERING, MANUFACTURING
引用次数: 0
摘要
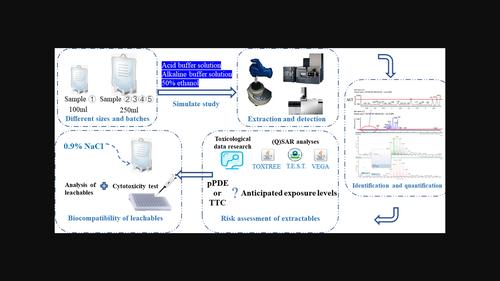
本研究利用超高效液相色谱法结合四极杆飞行时间质谱法和气相色谱-质谱法技术,对暴露于三种萃取剂的多批次非聚氯乙烯(PVC)软袋进行了分析。根据已建立的 30 多种不同聚合物添加剂的相对响应因子库,确定了大容量注射剂的分析评估阈值,并对 30 种萃取物进行了筛选和(半)定量分析。为了确定不同批次之间萃取物的差异,采用了主成分分析、热图分析和分层聚类分析等多元统计方法。此外,还对萃取物进行了综合风险评估,对浸出物进行了生物安全评估。研究结果显示,两种萃取物的浓度超过了既定的安全阈值。此外,根据两个 (Q)SAR 软件工具的一致结论,其中一种萃取物被认为具有诱变风险。值得注意的是,某些萃取物的含量在不同批次之间存在差异,尤其是1,3-双(3,5-二叔丁基-4-羟基苄基)-1,3,5-三嗪烷-2,4,6-三酮。这种差异导致各批次的风险水平不一致。不过,在非聚氯乙烯软袋中检测到的浸出物含量均在安全阈值范围内,而且细胞毒性评估结果一致为阴性。因此,这项研究为开展有关大容量肠外给药容器和其他形式药品包装中可萃取物的批次稳定性调查提供了宝贵的数据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Study of extractables and leachables of non-polyvinyl chloride soft infusion bags from different batches by analytical assessment threshold screening
This study utilized ultra-high-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry and gas chromatography–mass spectrometry techniques to analyze multiple batches of non-polyvinyl chloride (PVC) soft bags exposed to three extractants. Based on an established library of relative response factors for more than 30 different polymer additives, analytical evaluation thresholds for large volume injectables were determined, and 30 extractables were screened and (semi-)quantitatively analyzed. To identify variations in extractables between batches, multivariate statistical methods such as principal component analysis, heat map analysis, and hierarchical cluster analysis were employed. Additionally, a comprehensive risk assessment of extractables and a biosafety assessment of leachables were conducted. The results of the study revealed that the concentrations of two extractables exceeded the established safety threshold. Moreover, one of these extractables was deemed to pose a mutagenic risk based on the consistent findings of two (Q)SAR software tools. Notably, certain extractables exhibited discrepancies in their content between batches, with particular emphasis on 1,3-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-1,3,5-triazinane-2,4,6-trione. This discrepancy led to inconsistencies in risk levels across batches. However, the levels of leachables detected in the non-PVC soft bags were found to be within the safety threshold, and cytotoxicity assessments consistently yielded negative results for leachables. Consequently, this study provides valuable data for conducting batch stability investigations concerning extractables in large volume parenteral drug delivery containers and other forms of pharmaceutical packaging.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Packaging Technology and Science
工程技术-工程:制造
CiteScore
4.90
自引率
7.70%
发文量
78
审稿时长
>12 weeks
期刊介绍:
Packaging Technology & Science publishes original research, applications and review papers describing significant, novel developments in its field.
The Journal welcomes contributions in a wide range of areas in packaging technology and science, including:
-Active packaging
-Aseptic and sterile packaging
-Barrier packaging
-Design methodology
-Environmental factors and sustainability
-Ergonomics
-Food packaging
-Machinery and engineering for packaging
-Marketing aspects of packaging
-Materials
-Migration
-New manufacturing processes and techniques
-Testing, analysis and quality control
-Transport packaging
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


