靶向CD20/CD3的b细胞淋巴瘤双特异性T细胞接合物:2023 EHA年会上的最新进展
IF 2.8
3区 医学
Q2 PHARMACOLOGY & PHARMACY
Therapeutic Advances in Chronic Disease
Pub Date : 2023-12-04
eCollection Date: 2023-01-01
DOI:10.1177/20406223231215701
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
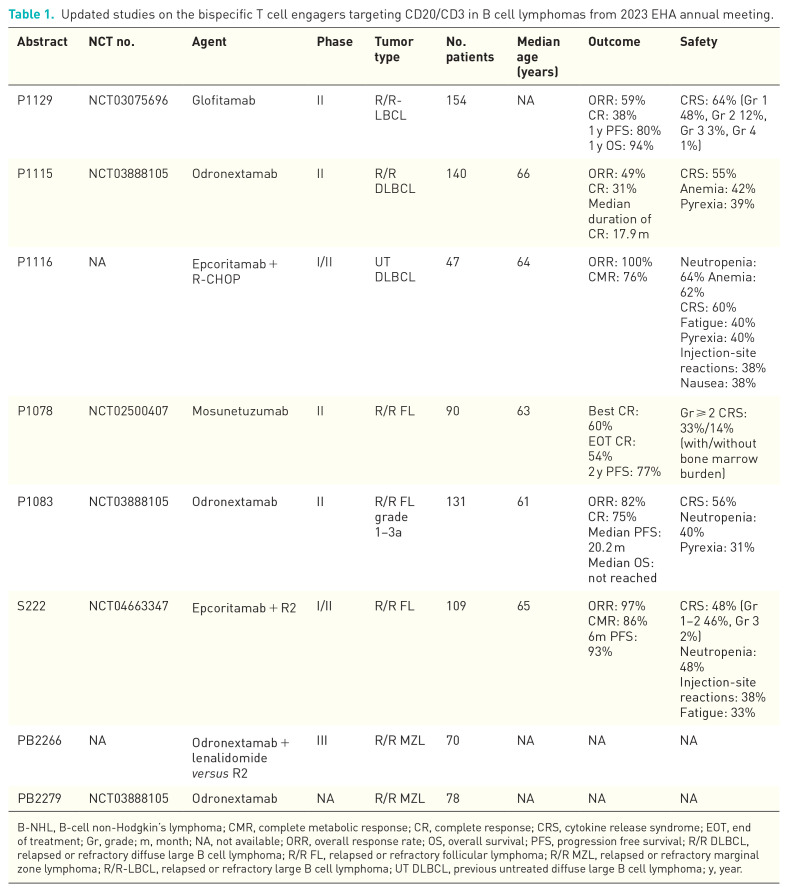

Bispecific T cell engagers targeting CD20/CD3 in B-cell lymphoma: latest updates from 2023 EHA annual meeting.
BiTE, has recently been approved in Europe for relapsed or refractory follicular lymphoma (R/R FL) treatment in adults who have received at least two prior systemic therapies. In a pivotal phase II study, 4 49 of 90 (54%) enrolled patients achieved CR at the end of treatment, with a 24-month PFS of 77%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Therapeutic Advances in Chronic Disease
Medicine-Medicine (miscellaneous)
CiteScore
6.20
自引率
0.00%
发文量
108
审稿时长
12 weeks
期刊介绍:
Therapeutic Advances in Chronic Disease publishes the highest quality peer-reviewed research, reviews and scholarly comment in the drug treatment of all chronic diseases. The journal has a strong clinical and pharmacological focus and is aimed at clinicians and researchers involved in the medical treatment of chronic disease, providing a forum in print and online for publishing the highest quality articles in this area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


