3-甲酰基-硫代氨基脲衍生物的合成
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
为了发现天然产物为基础的抗真菌候选物,制备了一系列新的3-甲酰基- n -吡唑基亚甲基吲哚的硫代氨基脲衍生物。部分衍生物具有良好的抗真菌活性。化合物I-2对弯孢菌的抑菌活性是前体吲哚的2.2倍。化合物Ⅰ-1的抗真菌活性是吲哚的1.7倍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
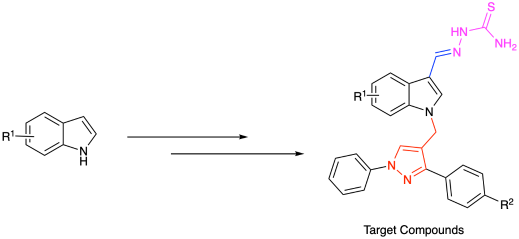
Synthesis of Thiosemicarbazone Derivatives of 3-Formyl-
In order to discover natural-product-based antifungal candidates, a series of new thiosemicarbazone derivatives of 3-formyl-N-pyrazolylmethyleneindoles were prepared. Some derivatives showed good antifungal activities. Against Curvularia lunata, compound I-2 showed 2.2 times antifungal activity compared to the precursor indole. Against Valsa mali, compound Ⅰ-1 exhibited 1.7 times antifungal activity compared to indole.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Heterocycles
化学-有机化学
CiteScore
1.50
自引率
0.00%
发文量
108
审稿时长
1 months
期刊介绍:
Since its inception in 1973 HETEROCYCLES has provided a platform for the rapid exchange of research in the areas of organic, pharmaceutical, analytical, and medicinal chemistry of heterocyclic compounds in addition to communications, papers, reviews, a special section of the journal presents newly-discovered natural products whose structure has recently been established.
Another section is devoted to the total synthesis of previously documented natural products with heterocyclic ring systems.
Due to the fact that the journal is able to publish articles within two months of receipt of the manuscripts, researchers in this field can obtain up-to-date information on heterocyclic research by reading Heterocycles regularly.
Audience: Organic and Physical Organic Chemists, Biochemists, Pharmacologists and Scientists studying heterocyclic compounds
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


