七熊果苷支链β-环糊精的合成
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文介绍了以β-环糊精(CD)为基础的熊果苷(4-羟基苯基β-葡萄糖苷)偶联物的合成,作为药物传递分子的模型。我们在其初级侧引入了三种七熊果苷支化CDs的变体。其中,两个结合的低聚(乙烯)链在其间隔中,以证明β-葡萄糖苷部分之间不同的空间特征的影响。由于β-CD在主侧的位阻位置,我们采用了高效的点击化学反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
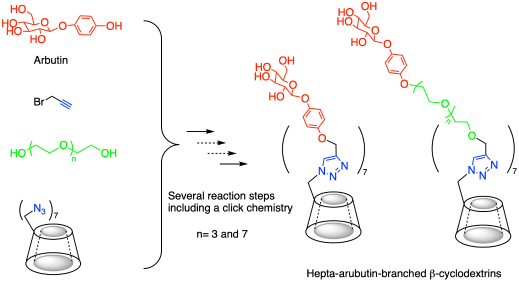
Synthesis of Hepta-arbutin-branched β-Cyclodextrins at Their Primary Sides
This paper describes the synthesis of β-cyclodextrin (CD) based arbutin (4-hydroxyphenyl β-glucopyanoside) conjugates as a model for drug delivery molecules. We introduced three variants of hepta-arbutin-branched CDs at their primary side. Of these, two incorporated oligo(ethylene)chains within their spacers to demonstrate the effect of varying spatial characteristics between the β-glucopyanoside moieties. Due to the sterically hindered position on the primary side of β-CD, we employed a highly efficient click chemistry reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Heterocycles
化学-有机化学
CiteScore
1.50
自引率
0.00%
发文量
108
审稿时长
1 months
期刊介绍:
Since its inception in 1973 HETEROCYCLES has provided a platform for the rapid exchange of research in the areas of organic, pharmaceutical, analytical, and medicinal chemistry of heterocyclic compounds in addition to communications, papers, reviews, a special section of the journal presents newly-discovered natural products whose structure has recently been established.
Another section is devoted to the total synthesis of previously documented natural products with heterocyclic ring systems.
Due to the fact that the journal is able to publish articles within two months of receipt of the manuscripts, researchers in this field can obtain up-to-date information on heterocyclic research by reading Heterocycles regularly.
Audience: Organic and Physical Organic Chemists, Biochemists, Pharmacologists and Scientists studying heterocyclic compounds
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


