太阳能光和超声波辅助快速Fenton氧化2,4,6-三氯苯酚:比较,优化和矿化
IF 2.7
4区 综合性期刊
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
氯酚是工业生产单位排放到水体中的持久性有机污染物。由于酚类废水的高稳定性和复杂性,传统的处理方法对其的处理是一个艰巨的过程。本研究采用光fenton法降解2,4,6-三氯苯酚。本研究开始对不同的调节参数如pH、氧化剂(H2O2)和Fe2+离子在不同浓度的批处理模式下进行优化和验证。在pH 3.0、Fe2+ 0.5 mM和H2O2 (10.0 mM)条件下,反应时间为6 min,三氯苯酚被完全降解。通过TOC分析仪和HPLC对模型污染物的矿化进行了研究。绘制响应面图来定义控制芬顿过程的过程之间的交互关系。在优化条件下,比较了Solar-Fenton、Sono-Fenton和Sono-Photo-Fenton (UV365)等不同fenton集成工艺对TCP的降解效果。其中,Solar-Fenton可在5 min内快速完全降解TCP。Fenton法、Solar-Fenton法、Sono-Fenton法、Sono-Photo-Fenton法和Photo-Fenton法的矿化效率分别为50%、98%、90%、75%和50%。结果表明,太阳能辅助Fenton是一种有潜力的高效降解三氯酚的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
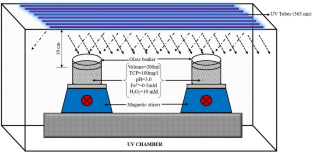
Solar light and ultrasound-assisted rapid Fenton’s oxidation of 2,4,6-trichlorophenol: comparison, optimisation, and mineralisation
Chlorophenols are the persistent organic contaminants released in the aquatic bodies by industrial manufacturing units. Treatment of phenolic wastewater is an arduous process for conventional treatment methods because of their high stability and complexity. The present study deals with degradation of 2,4,6-Trichlorophenol, using Photo-Fenton’s process. The study commences the optimisation and validation of different regulating parameters like pH, oxidant (H2O2), and Fe2+ ions at variable concentration in batch mode. At pH 3.0, Fe2+ 0.5 mM, and H2O2 (10.0 mM), complete degradation of trichlorophenol was observed within 6 min of reaction time. The mineralisation of the model pollutant was studied over TOC analyser and HPLC. Response Surface Plots were drawn to define the interactive relationship between process governing Fenton’s process. Under optimized conditions, different Fenton-integrated processes, such as Solar-Fenton, Sono-Fenton, and Sono-Photo-Fenton (UV365), were compared for degradation of TCP. Among all the processes, Solar-Fenton resulted in rapid and complete degradation of TCP within 5 min. The mineralisation efficiency of Fenton’s process, Solar-Fenton, Sono-Fenton, Sono-Photo-Fenton, and Photo-Fenton’s processes were 50%, 98%, 90%, 75%, and 50%, respectively. The results indicated Solar-assisted Fenton as potential and efficient approach toward degradation of Trichlorophenol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Rendiconti Lincei-Scienze Fisiche E Naturali
MULTIDISCIPLINARY SCIENCES-
CiteScore
4.10
自引率
10.00%
发文量
70
审稿时长
>12 weeks
期刊介绍:
Rendiconti is the interdisciplinary scientific journal of the Accademia dei Lincei, the Italian National Academy, situated in Rome, which publishes original articles in the fi elds of geosciences, envi ronmental sciences, and biological and biomedi cal sciences. Particular interest is accorded to papers dealing with modern trends in the natural sciences, with interdisciplinary relationships and with the roots and historical development of these disciplines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


