铜介导双硫酯合成2-取代苯并噻唑的高效途径及其抗菌活性研究
IF 2.2
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
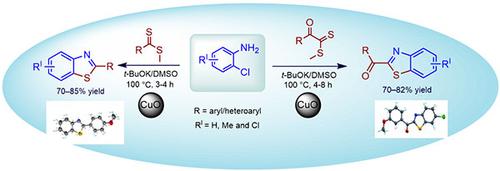
以铜为媒介,用二硫酯与2-氯苯胺缩合,一锅法合成了2-芳基/芳基苯并噻唑。该方法提供了良好的分离产率,并表现出广泛的官能团耐受性,可以容纳基底上的供电子和吸电子基团。对合成的一系列化合物进行了对肺炎克雷伯菌、铜绿假单胞菌和副伤寒沙门氏菌的抑菌活性评价。其中化合物4n、5q和5r对病原菌有明显的抑制作用。化合物5r在琼脂孔扩散试验和肉汤微量稀释试验中均表现出作为有效化合物的潜力。此外,化合物4n、5q和5r在10 mM浓度的结晶紫实验和MTT实验中都对病原体的生物膜形成有很强的抑制作用。这些发现突出了这些化合物有希望的抗菌和抗生物膜特性,表明它们作为潜在的治疗药物对所测试的病原体有进一步的研究潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An Efficient Copper-Mediated Route for the Synthesis of 2-Substituted Benzothiazoles from Dithioesters and Investigation of their Anti-bacterial Activities
An efficient one-pot synthesis of 2-aryl/aroyl benzothiazoles has been developed through copper-mediated condensation of 2-chloroaniline with dithioesters. The method provides good isolated yields and exhibits broad functional group tolerance, accommodating both electron-donating and electron-withdrawing groups on the substrate. A series of synthesized compounds was evaluated for their antibacterial activity against Klebsiella pneumoniae, Pseudomonas aeruginosa, and Salmonella paratyphi. Among the series, compounds 4n, 5q, and 5r exhibited a significant inhibitory effect against the tested pathogens. Compound 5r demonstrated potential as an effective compound in both the agar well diffusion assay and broth microdilution assay. Additionally, compounds 4n, 5q, and 5r displayed strong inhibitory effects on biofilm formation of the pathogens in both the Crystal violet assay and MTT assay at a concentration of 10 mM. These findings highlight the promising antimicrobial and anti-biofilm properties of these compounds, indicating their potential for further investigation as potential therapeutic agents against the tested pathogens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


