抗癌药物甲磺酸埃瑞布林C14-C26片段的聚合合成方法
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了通过将可从市售原料巴豆酸和1,4-丁二醇中获得的C14-C19片段与C20-C26片段偶联,以会聚的方式立体选择性合成布林C14-C26片段。该方法的关键步骤是Hosomi-Sakurai不对称烷基化、Maruoka烯丙化、Noyori还原、银催化的一锅重排和分子内环化本文章由计算机程序翻译,如有差异,请以英文原文为准。
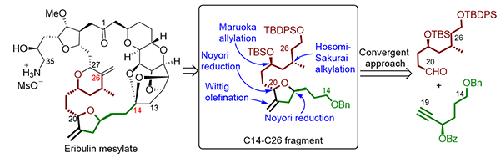
A convergent approach for the synthesis of C14-C26 fragment of anti-cancer drug eribulin mesylate
The stereoselective synthesis of C14-C26 fragment of eribulin is reported in a convergent way by coupling of fragment C14-C19 with fragment C20-C26 that are accessible from commercially available raw materials crotonic acid and 1,4-butanediol. The key steps involved in this practical approach are Hosomi-Sakurai asymmetric alkylation, Maruoka allylation, Noyori reduction, silver catalyzed one pot rearrangement and intramolecular cyclization
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


