过渡金属催化烷基亲电试剂Sonogashira交叉偶联反应的研究进展
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
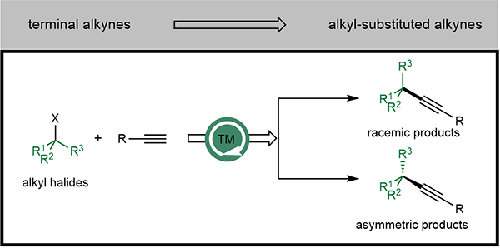
催化Sonogashira交叉偶联反应是一种高效和通用的方法,用于从现成的起始材料构建复杂的炔烃。尽管在这一领域取得了显著进展,但过渡金属催化的烷基亲电试剂Sonogashira交叉偶联反应的发展仍然有限。这种限制主要源于这些化合物的低反应性和明显倾向于β-H消除。为了克服这些挑战,研究人员研究了钯、镍和铜催化剂在烷基亲电试剂Sonogashira反应中的应用。此外,在实现亲电试剂的不对称Sonogashira交叉偶联反应方面取得了重大进展。这篇简短的综述概述了这一领域的最新突破。1简介2钯催化的烷基卤化物Sonogashira交叉偶联3镍催化的烷基卤化物Sonogashira交叉偶联4铜催化的烷基亲电试剂Sonogashira交叉偶联4.1铜催化的烷基亲电试剂外消旋Sonogashira交叉偶联4.2铜催化的烷基亲电试剂不对称Sonogashira交叉偶联5结论与展望本文章由计算机程序翻译,如有差异,请以英文原文为准。
Recent Advances in Transition-Metal-Catalyzed Sonogashira Cross-Coupling Reactions of Alkyl Electrophiles
Abstract Catalytic Sonogashira cross-coupling reactions represent an efficient and versatile approach for constructing complex alkynes from readily available starting materials. Despite notable progress in this field, the development of transition-metal-catalyzed Sonogashira cross-coupling reactions of alkyl electrophiles remains limited. This limitation primarily stems from the low reactivity and pronounced propensity of these compounds towards β-H elimination. To overcome these challenges, researchers have investigated the use of palladium, nickel, and copper catalysts for Sonogashira reactions of alkyl electrophiles. Furthermore, significant strides have been made in achieving asymmetric Sonogashira cross-coupling reactions of electrophiles. This short review provides an overview of recent breakthroughs in this area. 1 Introduction 2 Palladium-Catalyzed Sonogashira Cross-Coupling of Alkyl Halides 3 Nickel-Catalyzed Sonogashira Cross-Coupling of Alkyl Halides 4 Copper-Catalyzed Sonogashira Cross-Coupling of Alkyl Electrophiles 4.1 Copper-Catalyzed Racemic Sonogashira Cross-Coupling of Alkyl Electrophiles 4.2 Copper-catalyzed Asymmetric Sonogashira Cross-Coupling of Alkyl Electrophiles 5 Conclusions and Perspectives
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


