光照射下羧胺加速碘芳烃的化学选择性硼化反应
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
卤代芳烃的硼化反应是合成卤代芳烃的重要方法之一。一般来说,卤化芳烃的硼化反应通过使用过渡金属、光氧化还原催化剂或基本条件,在空间阻力较小的对位或间位上顺利进行。本研究报道了在可见光照射下邻碘芳烃含甲酰胺的正交特异性和化学选择性硼化反应。当含有C-I和C-X键的卤代烃作为底物时,另一个C-X键(非邻位键)在反应过程中保持完整。机理研究表明,该反应成功的关键是生成二硼桥式五元环作为过渡态,其中二硼桥式五元环与处于过渡态的苯环由于邻位碘原子的位排斥力而相互垂直。这种化学选择性适用于硼酸基块的合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
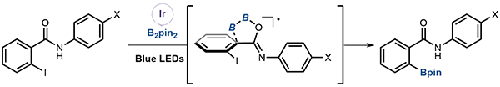
Carboxamide-accelerated Chemoselective Borylation of Iodoarenes under Photoirradiation
Borylation of haloarene is one of the most important methodologies to synthesize borylated arenes. Generally, borylation of haloarene occurs smoothly at sterically less-hindered para- or meta-position by using a transition metal, a photoredox catalyst, or basic conditions. This study reports on ortho-specific and chemoselective borylation of ortho-iodoarene possessing carboxamide under visible-light irradiation. When a haloarene containing both C–I and C–X bonds is employed as a substrate, another C–X bond (not ortho) remains intact during the reaction. Mechanistic studies revealed that the key to success of this reaction is to generate diboron-bridged five-membered ring as a transition state, in which the diboron-bridged five-membered ring and the benzene ring in the transition state are perpendicular to each other owing to steric repulsion by the iodine atom at the ortho-position. This chemoselectivity is suitable for the synthesis of borylated building blocks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


