研究偶氮苯部分与芳香氨基酸色氨酸的相互作用
IF 1.5
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
偶氮苯是一类在光照射下可以异构化的化合物。这些分子可以改变各种材料的物理、化学和生物特性。它们能够以时间和空间精度控制蛋白质的结构和功能。在这项工作中,我们研究了偶氮苯和芳香氨基酸之间可能的相互作用。我们假设芳香氨基酸,如色氨酸,当与偶氮苯偶联时,会显示出改变的光化学性质。当在365 nm或465 nm照射时,分子现在缺乏通常特征的光开关能力,并且在365 nm处可见荧光。据我们所知,这是第一个表明初级蛋白质结构可以影响光开关活性的证据。从这项研究中获得的知识将有助于进一步了解偶氮苯,因为它们被用于生物分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
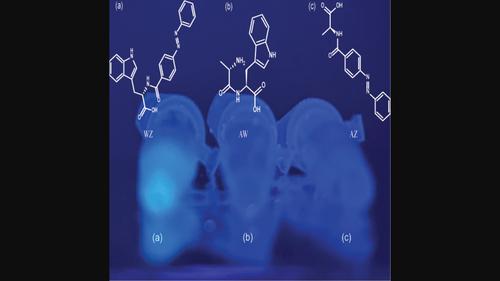
Investigating the interaction of azobenzene moiety on the aromatic amino acid tryptophan
Abstract Azobenzenes are a series of compounds that can be isomerized upon irradiation with light. These molecules can modify the physical, chemical, and biological properties of a diverse range of materials. They can control protein structure and function with temporal and spatial precision. In this work, we investigated the possible interaction between azobenzene and aromatic amino acids. We hypothesized that aromatic amino acids, such as tryptophan, would show altered photochemical properties when conjugated with azobenzene. When irradiated at either 365 nm or 465 nm, the molecule now lacks the usually characteristic photoswitch capabilities and is visibly fluorescent at 365 nm. To our knowledge, this is the first evidence to suggest that primary protein structure could affect photoswitch activity. The knowledge gained from this research will help to further the understanding of azobenzenes as they are used in biomolecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


