膨润土含铁组分硫化物消耗量的测定
IF 2.1
4区 地球科学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
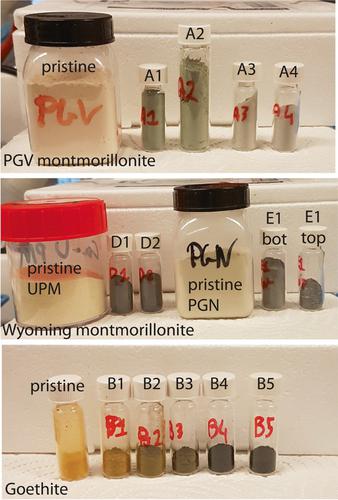
用过的核燃料的地质储存库使用膨润土作为缓冲,以保护限制放射性物质的金属容器。地下水中可能存在的硫酸盐还原细菌,在膨润土-寄主岩石界面或最终在膨润土内部可能产生硫化物,这对金属罐,特别是铜罐构成潜在威胁。膨润土可以作为潜在的硫化物清除剂。然而,对于潜在的机制、硫化物消耗的最大程度以及储存库条件下对膨润土结构的潜在影响,人们知之甚少。在目前的研究中,浓缩(4-150 mM)硫化物溶液与六种天然含铁膨润土、各种纯化的膨润土含铁成分(一系列纯化的蒙脱土和三种铁(氧)氧化物)以及一种合成混合物在pH值7至13范围内反应长达1.5个月。溶液采用比色法测定硫化物和多硫化物浓度,固体采用57 Fe Mössbauer光谱法测定铁形态。在粘土样品中观察到重要的硫化物消耗与结构铁的减少。并不是所有的粘土结构铁都与硫化物发生反应;活性结构铁的比例与粘土结构和ph值有关。固体样品中铁在溶液中存在过量硫化物时,粘土结构铁是主要的反应物,而与铁(氧)氧化物的反应在很大程度上被抑制。根据膨润土主要粘土组分的硫化物氧化能力,区分了3个膨润土基团。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Determination of Sulfide Consumption by Fe-bearing Components of Bentonites
Abstract Geologic repositories for spent nuclear fuel use bentonite as a buffer to protect the metallic containers confining the radioactive material. Sulfate-reducing bacteria, which may be present in groundwater, at the bentonite–host rock interface or eventually within the bentonite may produce sulfide, representing a potential threat for the metallic canisters, particularly copper. Bentonites can act as potential sulfide scavengers. Little is yet known, however, regarding the underlying mechanisms, the maximum extent of sulfide consumption, and the potential impacts on bentonite structure under repository conditions. In the current study, concentrated (4–150 mM) sulfide solutions were reacted in batch experiments with six natural Fe-bearing bentonites, with various purified Fe-bearing components of bentonite (a series of purified montmorillonites and three iron (oxyhydr)oxides), and with one synthetic mixture, for up to 1.5 months at pH values ranging from 7 to 13. The solutions were analyzed by colorimetry to determine sulfide and polysulfide concentrations and the solids were analyzed by 57 Fe Mössbauer spectrometry to determine iron speciation. Important sulfide consumption coupled with a reduction of structural Fe in the clay samples was observed. Not all clay structural Fe was reactive toward sulfide; the proportion of active structural Fe depended on the clay structure and pH. In the presence of excess sulfide in solution regarding Fe in the solid sample, the clay structural Fe was found to be the main reactant while the reaction with iron (oxyhydr)oxides was largely inhibited. Three bentonite groups were distinguished, based on the sulfide oxidation capacity of their main clayey component.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Clays and Clay Minerals
地学-地球科学综合
CiteScore
3.40
自引率
9.10%
发文量
46
审稿时长
3 months
期刊介绍:
Clays and Clay Minerals aims to present the latest advances in research and technology concerning clays and other fine-grained minerals, including but not limited to areas in agronomy, ceramics, colloid chemistry, crystallography, environmental science, foundry engineering, geochemistry, geology, medicinal chemistry, mineralogy, nanoscience, petroleum engineering, physical chemistry, sedimentology, soil mechanics, and soil science. Clays and Clay Minerals exists to disseminate to its worldwide readership the most recent developments in all of these aspects of clay materials. Manuscripts are welcome from all countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


