HNO3/HCl促进2-炔基双芳基与二硫化物环化无金属合成9-亚砜基菲
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究了2-炔基双芳基与二硫化物的无金属硫代环化反应。这种温和而高效的方法在廉价的HNO3/HCl的促进下,具有广泛的底物范围和高官能团耐受性,可提供一系列相应的9-亚砜基菲。反应中使用了二硫试剂的两个硫化物基团。本文章由计算机程序翻译,如有差异,请以英文原文为准。
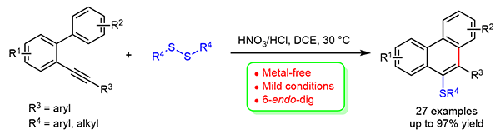
Metal-Free Synthesis of 9-Sulfenylphenanthrenes via HNO3/HCl Promoted Annulation of 2-Alkynyl Biaryls with Disulfides
A metal-free thiolative annulation of 2-alkynylbiaryls with disulfides has been developed. This mild and efficient approach was promoted by inexpensive HNO3/HCl and afforded a range of corresponding 9-sulfenylphenanthrenes in good to excellent yields with a broad substrate scope and high functional group tolerance. Both sulfide groups of the disulfide reagent were used in the reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


