膦配体在镍催化烯烃迁移氢烷基化中的作用
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
催化烯烃加氢烷基化反应为以易得且价格低廉的烯烃原料为原料,经烯烃加氢金属化,再经交叉偶联机理合成C(sp3)中心提供了一种有效的方法。该领域的主要任务之一是开发不同的配体来实现区域选择性控制。本文报道了镍-三苯基膦催化烯基酰胺远端氢烷基化反应制备α-支胺的研究。各种烯烃和烷基碘化物是合适的底物,可以提供具有优异区域选择性(20:1区域异构体比)的所需产品。密度泛函理论计算揭示了反应机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
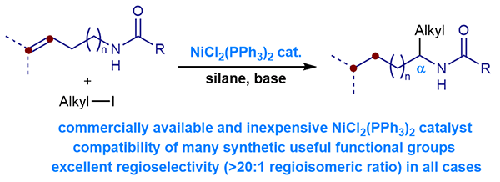
Phosphine Ligand Effects in Nickel-Catalyzed Alkene Migratory Hydroalkylation
Catalytic alkene hydroalkylation has provided an efficient method for synthesizing C(sp3) centers, from readily available and inexpensive alkene starting materials through alkene hydrometallation and then cross-coupling mechanism. One of the major tasks in this field is to develop diverse ligands to achieve regioselective control. Herein, we report the investigation of nickel–triphenylphosphine catalyzed remote hydroalkylation of alkenyl amides to access α-branched amines. Various alkenes and alkyl iodides are suitable substrates to deliver desired products with excellent regioselectivities (>20:1 regioisomeric ratio). Density functional theory calculations reveal the reaction mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


