铑(III)在吲哚中催化C-H活化:综合报告(2017-2022)
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
摘要在合成有机化学领域,过渡金属催化C-H定向活化是合成天然产物、有机材料和基本有机构件的一种杰出而有效的方法。值得注意的是,这种策略近年来取得了显著的进展,特别是在各种基底类别的应用中,包括必不可少的吲哚支架。吲哚是有机化学中备受追捧的靶标。吲哚的意义超出了它在全合成和药物发现中的应用。它也是开发药剂、农用化学品和材料的重要工具。通过靶向吲哚,合成化学家可以获得广泛的生物活性化合物,这为药物开发和化学生物学研究开辟了新的途径。通过使用C-H活化作为多功能功能化平台,开发了一个全面的工具包,极大地帮助了结构变化吲哚的合成。本文综述了铑在吲哚支架C2、C4和C7位点催化C-H活化方面的最新突破。这些发展代表了该领域的重大进展,并具有在吲哚基化合物合成方面进一步取得进展的良好潜力。1简介2铑催化C-H活化的研究进展3 Rh -催化C-H活化的一般机理介绍4吲哚的直接C-H功能化4.1吲哚的C2活化4.2吲哚的C4活化4.3双C-H活化策略4.4吲哚的C7活化5结论本文章由计算机程序翻译,如有差异,请以英文原文为准。
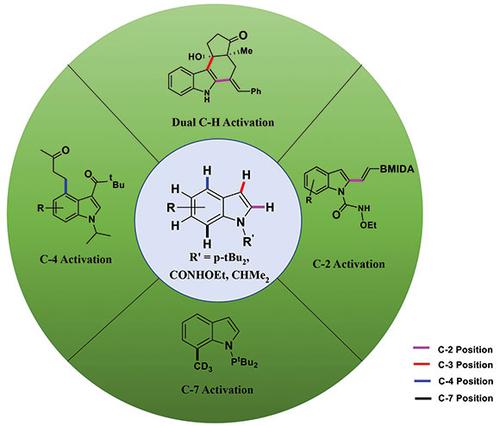
Rhodium(III)-Catalyzed C–H Activation in Indole: A Comprehensive Report (2017–2022)
Abstract In the realm of synthetic organic chemistry, the catalysis of directed C–H activation by transition metals is an outstanding and efficient method for the synthesis of natural products, organic materials, and fundamental organic building blocks. Notably, this strategy has experienced remarkable advances in recent years, particularly in its application to various substrate classes, including the essential indole scaffold. Indole is a highly sought-after target in organic chemistry. The significance of indole extends beyond its use in total synthesis and drug discovery. It also serves as an important tool in the development of pharmaceutical agents, agrochemicals, and materials. By targeting indole, synthetic chemists can access a wide range of bioactive compounds, which opens new avenues for drug development and chemical biology research. The synthesis of structurally varied indoles has been greatly aided by the development of a comprehensive toolkit made possible by the use of C–H activation as a versatile functionalization platform. This review highlights the latest breakthroughs in rhodium-catalyzed C–H activation at the C2, C4, and C7 positions of the indole scaffold. These developments represent significant progress in the field and hold promising potential for further advances in the synthesis of indole-based compounds. 1 Introduction 2 The Development of Rhodium-Catalyzed C–H Activation 3 General Mechanistic Introduction to Rh(III)-Catalyzed C–H Activation 4 Direct C–H Functionalization of Indoles 4.1 C2 Activation of Indoles 4.2 C4 Activation of Indoles 4.3 Dual C–H Activation Strategy 4.4 C7 Activation of Indoles 5 Conclusion
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


