Diels-Alder/Tsuji-Trost提取Kopsia生物碱路线的检验
IF 2.2
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
摘要研究了Diels-Alder环加成和Tsuji-Trost烯丙化反应序列在kopsinine及相关Kopsia生物碱合成中的应用。本文介绍了两个合成方向的研究结果:1)Diels-Alder步骤所需的适当取代的二烯的合成;2)无侧链条件下关键反应序列的模型研究与优化。在第一个方向上,详细介绍了具有挑战性的侧链引入四碳氢酮及其硅基化,导致罕见但无效的葡萄clisen环化,以及成功的Mannich/Mukaiyama aldol序列。在第二个方向上,揭示了与丙烯酸烯丙酯的内选择性Diels-Alder反应和提供不正确立体化学的Tsuji-Trost烯丙基化。两种二烯与炔的相互作用通过Alder-Rickert反应生成咔唑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
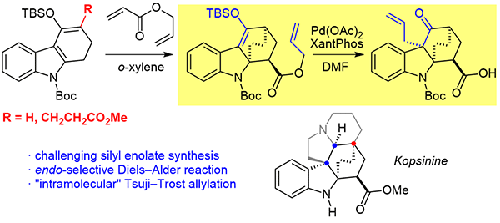
Examination of Diels-Alder/Tsuji-Trost Route towards Kopsia Alkaloids
Abstract A reaction sequence of Diels–Alder cycloaddition and Tsuji–Trost allylation was examined in terms of its application to the synthesis of kopsinine and the related Kopsia alkaloids. Results of the studies in two synthetic directions are presented herein: 1) synthesis of the properly substituted diene, required for the Diels–Alder step; and 2) model studies and optimization of the key reaction sequence in the absence of side-chain. Details on the challenging introduction of the side-chain into tetrahydrocarboline ketone and its silylation, resulting in rare but unproductive vinylogous Claisen cyclization, and the successful Mannich/Mukaiyama aldol sequence are disclosed in the first direction. In the second direction, the endo-selective Diels–Alder reaction with allyl acrylate and Tsuji–Trost allylation providing incorrect stereochemistry are disclosed. Interaction of both dienes with an alkyne provides carbazoles via Alder–Rickert reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


