金属负载ZrO2催化剂催化氧化1,2-二氯苯
IF 2.8
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
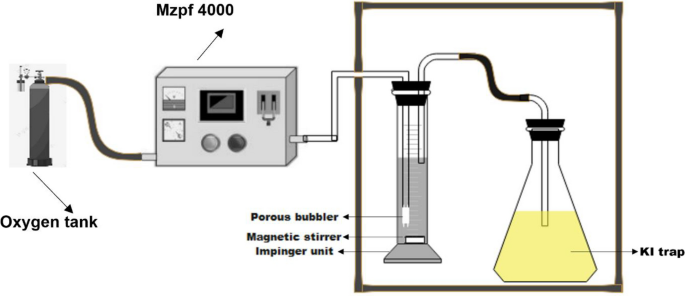
对环境不友好的氯化有机物的催化氧化是将这些有机化合物完全矿化的有力工具。在本研究中,研究了氧化锆负载金属(Mn, Ni, V和Fe)作为1,2-二氯苯氧化的催化剂。采用湿浸渍法制备了氧化锆催化剂上的金属载体。采用FT-IR、XRD、SEM-EDX、TEM、BET和ICP等方法对催化剂材料进行了表征。通过ICP-OES和EDX分析证实,金属以不同wt%、2.5、5、7.5和10的比例加载在ZrO - 2载体上。结果表明,负载在催化剂上的金属的XRD衍射峰均属于单斜相,而属于金属氧化物的衍射峰较少。当金属被加载在二氧化锆支架上时,BET表面积显著减小。ZrO 2的BET表面积为4.91 m2 /g,当负载2.5%和7.5% wt% Fe时,ZrO 2的BET表面积分别为4.48和3.09 m2 /g。所有1,2-二氯苯的臭氧化反应在冲击装置中进行24 h,在臭氧化3、6、9、12、15、18和24小时后收集等分,采用FT-IR和GC-MS分析。发现7.5% Fe/ zro2对1,2-二氯苯转化为多氯酸和3,4-二氯-2,5-呋喃二酮具有较高的催化作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Catalytic Oxidation of 1,2-Dichlorobenzene over Metal-Supported on ZrO2 Catalysts
Abstract The catalytic oxidation of environmentally unfriendly chlorinated organics is a powerful tool that can be used to completely mineralize these organic compounds. In this study, metals (Mn, Ni, V, and Fe) supported on zirconia have been investigated as catalysts in the oxidation of 1,2-dichlorobenzene. Metal supported on zirconia catalysts were prepared using the wet impregnation method. The catalyst materials were characterized by FT-IR, XRD, SEM–EDX, TEM, BET, and ICP methods. Metals were loaded on ZrO 2 support at varied wt%, 2.5, 5, 7.5, and 10 which were confirmed by ICP-OES and EDX analysis. The XRD patterns of all the metals loaded on ZrO 2 catalysts showed diffraction peaks that belong to the monoclinic phase of ZrO 2 and few that are attributed to the oxides of the metals. The BET surface areas showed a significant decrease as the metal was loaded on ZrO 2 support. The BET surface area of ZrO 2 was 4.91 m 2 /g and when 2.5 and 7.5 wt% Fe was loaded on ZrO 2 the BET surface areas were 4.48 and 3.09 m 2 /g, respectively. All the ozonation reactions of 1,2-dichlorobenzene were conducted in an impinger unit for 24 h. The aliquots were collected after 3, 6, 9, 12, 15, 18, and 24 of ozonation and analyzed using FT-IR and GC–MS analyses. The 7.5% Fe/ZrO 2 was found to have a relatively high catalytic effect toward the conversion of 1,2-dichlorobenzene into mucochloric acid and 3,4-dichloro-2,5-furandione.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Topics in Catalysis
化学-物理化学
CiteScore
5.70
自引率
5.60%
发文量
197
审稿时长
2 months
期刊介绍:
Topics in Catalysis publishes topical collections in all fields of catalysis which are composed only of invited articles from leading authors. The journal documents today’s emerging and critical trends in all branches of catalysis. Each themed issue is organized by renowned Guest Editors in collaboration with the Editors-in-Chief. Proposals for new topics are welcome and should be submitted directly to the Editors-in-Chief.
The publication of individual uninvited original research articles can be sent to our sister journal Catalysis Letters. This journal aims for rapid publication of high-impact original research articles in all fields of both applied and theoretical catalysis, including heterogeneous, homogeneous and biocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


