5′- o -甲基-14-羟基蜜环烷:一种新的蜜环烷型倍半萜
IF 3
3区 生物学
Q3 MYCOLOGY
引用次数: 0
摘要
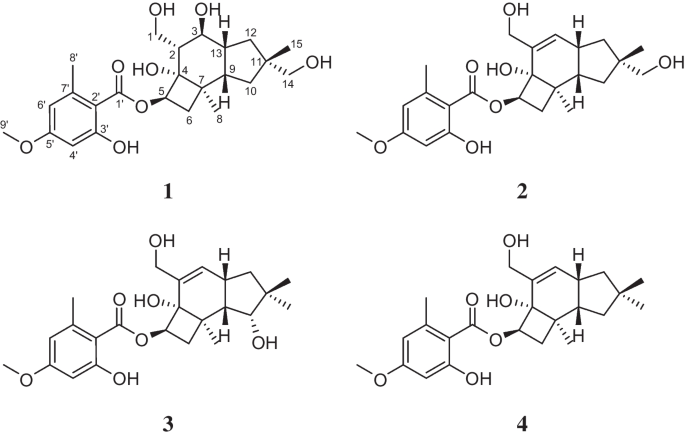
原illudene型倍半萜芳基酯是迄今为止仅从蜜环菌属(Agaricomycetes, Physalacriaceae)成员中分离到的一种独特而多样的化合物。在此,我们描述了5 ' - O -甲基-14-羟基蜜环烷(1)的分离和结构表征,这是一种新的蜜环烷型衍生物,从Guyanagaster necrorhiza (CBS 138623)的深层培养中获得,与已知的同系物melleolide G (2), melleolide B(3)和10- de羟基-melleolide B(4)一起。ROESY数据和偶联常数确定其相对构型为1,而通过与10-羟基-5′-甲氧基-6′-氯胺环烷的ECD谱比较,确定其常见的绝对构型(5)。此外,根据观察到的ROESY相关性,对千层内酯G(2)的构型进行了修正。这是首次从蜜环菌亲缘关系较近的Guyanagaster属中分离到原illudene型倍半萜芳基酯。在对几种酵母、真菌和细菌的一系列稀释试验中,以及对不同细胞系的细胞毒性试验中,对1 - 4进行了生物活性评估。化合物4对枯草芽孢杆菌、金黄色葡萄球菌和毛霉菌有中等活性。此外,1、3、4对小鼠成纤维细胞系L929和宫颈癌细胞系KB3.1表现出较弱的细胞毒作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
5′-O-methyl-14-hydroxyarmillane, a new armillane-type sesquiterpene from cultures of Guyanagaster necrorhiza
Abstract Protoilludene-type sesquiterpene aryl esters are a unique and very diverse compound class that were exclusively isolated from members of the genus Armillaria ( Agaricomycetes , Physalacriaceae ) up to this point. Herein, we describe the isolation and structural characterization of 5′- O -methyl-14-hydroxyarmillane ( 1 ), a new armillane-type derivative, that was obtained from submerged cultures of Guyanagaster necrorhiza (CBS 138623) together with the known congeners melleolide G ( 2 ), melleolide B ( 3 ), and 10-dehydroxy-melleolide B ( 4 ). ROESY data and coupling constants assigned the relative configurations of 1 , while common absolute configurations were confirmed from comparison of its ECD spectrum to the one of 10-hydroxy-5′-methoxy-6′-chloroarmillane ( 5 ). Additionally, the configuration of melleolide G ( 2 ) was revised based on observed ROESY correlations. It is the first time that protoilludene-type sesquiterpene aryl esters were isolated from another genus, namely, Guyanagaster , that is closely related to Armillaria . 1 – 4 were evaluated for their biological activities in a serial dilution assay against several yeast, fungi, and bacteria, as well as in a cytotoxicity assay against different cell lines. Compound 4 was moderately active against Bacillus subtilis , Staphylococcus aureus , and Mucor hiemalis . Furthermore, 1 , 3 , and 4 showed weak cytotoxic effects against the mouse fibroblast cell line L929 and the cervix carcinoma cell line KB3.1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mycological Progress
生物-真菌学
CiteScore
4.50
自引率
8.30%
发文量
94
审稿时长
6-12 weeks
期刊介绍:
Mycological Progress publishes papers on all aspects of fungi, including lichens. While Review Papers are highly welcome, the main focus is on Research Articles on
Taxonomy and Systematics
Evolution
Cell Biology
Ecology
Biotechnology
Pathology (plants, animals, humans)
Manuscripts on current methods applied in, e.g., morphology, anatomy, ultrastructure (TEM, SEM), genetics, molecular biology, chemistry, and physiology will also be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


