作为神经保护药物的新型肉桂衍生物的合成、晶体结构、Hirshfeld 表面分析和生物活性
IF 2.4
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
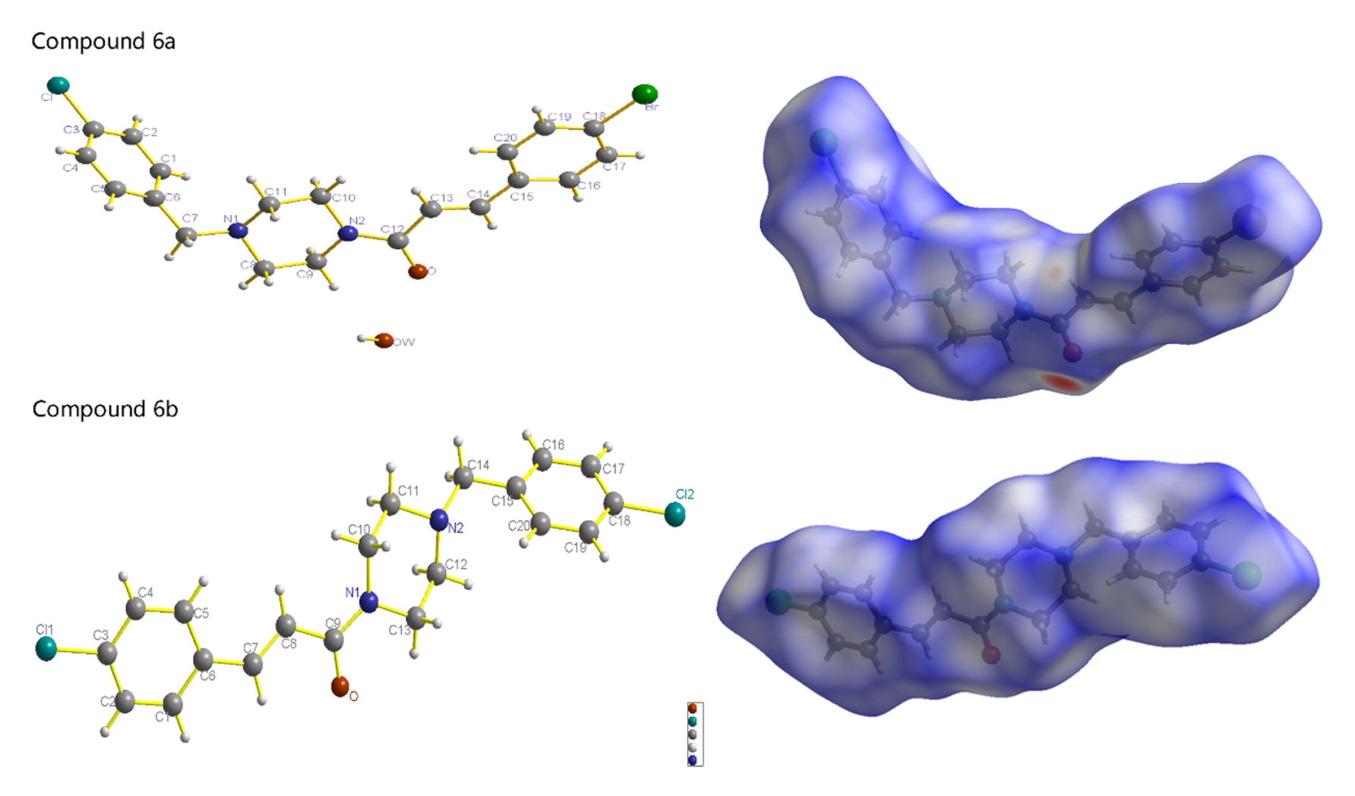
从中国传统药材 Scrophularia buergeriana 中提取的化合物所含的对甲氧基肉桂酰基具有显著的神经保护作用。以对甲氧基肉桂酰基为基础,设计并合成了两种肉桂酸衍生物,即(E)-3-(4-溴苯基)-1-(4-(4-氯苄基)哌嗪-1-基)丙-2-烯-1-酮(6a)和(E)-1-(4-(4-氯苄基)哌嗪-1-基)-3-(4-氯苯基)丙-2-烯-1-酮(6b)。对目标化合物进行了高分辨率质谱、1H NMR 光谱、13C NMR 光谱和单晶 X 射线衍射分析。单晶 X 射线衍射结果表明,化合物 6a 结晶于单斜晶系,化合物 6b 结晶于三斜晶系,它们都具有叠层三维结构。Hirshfeld表面表明,化合物6a和6b存在H...H、O...H、C...H和π...π分子间相互作用。此外,还进一步评估了它们对谷氨酸诱导的 PC12 细胞损伤的神经保护作用。这些结果表明,目标化合物对谷氨酸诱导的 PC12 细胞神经毒性具有强效活性。体内实验表明,大剂量的 6a 和 6b 化合物对双侧颈总动脉结扎具有良好的保护作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, Crystal Structure, Hirshfeld Surface Analyses and Biological Activity of Novel Cinnamide Derivatives as Neuroprotective Drugs
The p-methoxycinnamoyl group contained in the compounds extracted from Scrophularia buergeriana, which is a traditional medicinal herb in China, exhibits a remarkable neuroprotective effect. Based on the p-methoxycinnamoyl group, these two cinnamide derivatives, namely, (E)-3-(4-bromophenyl)-1-(4-(4-chlorobenzyl)piperazin-1-yl)prop-2-en-1-one (6a) and (E)-1-(4-(4-chlorobenzyl)piperazin-1-yl)-3-(4-chlorophenyl)prop-2-en-1-one (6b), were designed and synthesized. The target compounds were represented by High-resolution mass spectra, 1H NMR spectra, 13C NMR spectra, and single-crystal X-ray diffraction. According to the results of single-crystal X-ray diffraction, indicating that compound 6a crystallizes in the monoclinic system and compound 6b crystallizes in the triclinic system, and they both have a stacked-layer 3-D structure. Hirshfeld surface indicated that compounds 6a and 6b exist H…H, O…H, C…H, and π…π intermolecular interactions. Furthermore, their neuroprotective effects were further evaluated against glutamate-induced PC12 cell injury. These results indicate that the target compounds exhibit potent activities against glutamine-induced neurotoxicity in PC12 cells. In vivo experiments demonstrated that compounds 6a and 6b have good protective effects in high doses on bilateral common carotid artery ligation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polycyclic Aromatic Compounds
化学-有机化学
CiteScore
3.70
自引率
20.80%
发文量
412
审稿时长
3 months
期刊介绍:
The purpose of Polycyclic Aromatic Compounds is to provide an international and interdisciplinary forum for all aspects of research related to polycyclic aromatic compounds (PAC). Topics range from fundamental research in chemistry (including synthetic and theoretical chemistry) and physics (including astrophysics), as well as thermodynamics, spectroscopy, analytical methods, and biology to applied studies in environmental science, biochemistry, toxicology, and industry. Polycyclic Aromatic Compounds has an outstanding Editorial Board and offers a rapid and efficient peer review process, as well as a flexible open access policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


